(J. Tesarčík, Z. Svobodová)
The ways of prevention and contingently of medical treatment of fish are very specific and often different from those in warm-blooded animals. They require a thorough knowledge of the environment of fish. Preventive arrangements are consisting of complicated set of treatments elaborated on the base of a good knowledge of the aetiology of disease and a host (fish) biology. It concerns the elimination or restriction of infection (invasion) sources and the possibilities of its further expansion likewise the enhancement of condition of fish organism in the way to be able to withstand the infection (invasion). The prevention is of basic importance in diseases elimination. No specific therapeutics were developed for a number of diseases up to now and the result of the application of effective, experimentally verified medicaments, is often reversely affected by the operational conditions and/or the technology of rearing. The medical treatment becames economically unrenumerative in this way.
In addition, some treatments cannot be performed in certain periods, e.g. in growing season, during the wintering, or in some fish culture units (e.g. large ponds).
That is why it is much more important to prevent from the diseases than to recover them. The effective preventive treatments are to be applied above all in specialized fish culture units with closed warm water system, in early fish fry rearing, hatcheries, trout farms, wintering ponds and storage reservoirs.
Generally accepted and effective principles are as follows:
a) Providing water sources free of pathogens
Underground waters are the most suitable water sources free of pathogens. These sources are limited both for trout farms and hatcheries and for other special fish culture units at present. The surface water from rivers and channels is used as the source of inflow water in most cases. In these situations, suitable filters can partially reduce the numbers of invasion stages of parasites in inflow water, above all when supplying smaller reservoirs with intensive culture. Bars are usually placed before these filters to separate rough particles. Sand filters are consisted of a set of sedimentation divisions terminated by filter with fibre and sand. These type of filters catch above all the heavier parasite stages unable to move actively (e.g. spores). Lower efficiency is registered in elimination of moving parasites like e.g. infusorians.
The water from the pond with fish stock is quite unsuitable for these purposes (esp. as the source of inflow water for trout farms, hatcheries and units for early fish fry stages).
Chemical treatment of inflow water is an emergency arrangement with often undesirable parallel affects. Disinfection of the water entering fish culture units by UV radiation is not still an usual way although it can be considered as the simple method how to destroy viruses, bacteria and moulds germs. Since the inflow water from rivers and channels is slightly turbid and contents a number of suspended solids and dissolved compounds, the disinfective efficiency of UV radiation is markedly reduced in these situations.
It is very profitable to supply the individual ponds and/or reservoirs independently, not throughflowly. The water from each pond or reservoir should be drained separately and should not flow into any other. Especially quarantine ponds and other reservoirs can be separated by this way.
b) Protection from the transfer of pathogens
This principle means above all the transfer of pathogens by uncontrolled transport of fish and spawns. The transport of fish with unknown health condition is to be avoided in principle. All transported fish are to be accompanied by veterinary certificate confirming that fish were examined before transporting them, they are healthy and originate from the environment in which no important transfer diseases appear. The list of these diseases is precisely stated in veterinary instructions. Except of the internal survey for each country also the list of diseases stated in international codex is obligatory for veterinary service. This list is currently specified with the development of diagnostic methods and improvement of knowledge about individual fish diseases. Some viral and bacterial diseases can be transfered also by spawns. Their transport must be completed by the same veterinary certificate like fish transport from this reason.
Fish introduced from other territories must be subjected to quarantine for one year regardless if native or extraneous species. The duration of quarantine can be prolongated e.g. in the case of fish imported from abroad until the period of 3 years. Prolongated period of quarantine is of special importance especially in spawners predestined for further reproduction of imported species.
The selfsustaining in stock production in individual farms and similar organizations is a significant way of prevention from dissemination of fish diseases. Only fish previously examined, free of diseases and relevantly treated by medicinal baths are to be stocked into ponds and fish culture units. The stocking of fry originating from semi-artificial and artificial spawning not contacted with fish of higher age categories also minimizes the danger of infection.
The prevention from introduction of coarse fish into ponds and fish culture units is the other important arrangement protecting the stock against transfer of pathogens. These fish are above all the source of ectoparasites, dangerous especially in the period of decreased resistence of fish. Except of this they can transfer also some other pathogens which can result in heavy losses in important fish species. Adequate bars and filters can serve for prevention from coarse fish penetration.
The protection of piscivorous birds to step into fish culture units (esp. trout farms) is the prevention limiting the expansion of some fish diseases. Protective nets are used to prevent the birds from running in. The numbers of piscivorous birds are regulated in localities where overpopulated.
Preventive control of snails (Lymnaea sp.) as intermediate hosts of some fish parasites can be performed by biological (introduction of black carp - Myelopharyngodon piceus or 3-years-old tench Tinca tinca), mechanical (placing nets in the inflow), physical (drying and freezing of the bottom) and chemical (application of molluscocides) ways.
Safe and harmless removing of dead fish is a significant way how to prevent from further transfer of fish pathogens. Fresh or slightly decayed dead fish are decontaminated in the nearest veterinary facility. Lower masses of dead fish are to be burnt or burried into deep pits (aprox. 2 m) in distance of at least 20 m from the pond bank. The bottom of this pit and dead fish must be covered by burnt or chlorinated lime. The layer of at least 60 – 80 cm of the soil must cover the content of a pit.
c) Disinfection of ponds, fish culture units and equipment; winter freezing and summer drying of ponds
Disinfection is of a big importance in prevention and elimination of fish diseases. Preventive disinfection protects the fish stocks against pathogens. Hygiene of environmental conditions for fish is improved by this way. Focal disinfection is performed for control of the focus of dangerous fish disease.
Natural physical phenomena are fully used for disinfection in intensive fish culture due to their ecomical convenience. It concerns the drying and freezing of the pond bottom. The most of pathogens die after perfect drying of the pond bottom when its relative moisture had dropped on 10 – 15 %. The perfect freezing of the wet places and sun radiation (above all by its UV rays) have a very favourable effect in our conditions. The influence of these natural physical phenomena is exploited by summer drying and winter freezing of water reservoirs (ponds). Summer drying is a radical, long-term intervention during which all pathogens are controlled due to the perfect drying of the pond bottom. The aim of winter drying is to destroy the pathogens by freeze. It safely leads to destruction of leeches (Piscicola geometra), fish lice (Argulus sp.), predatory larvae of water insects, eggs and spores of parasites and also other pathogens. Employing the natural ways for disinfection has an disadvantage in usually long-term duration (a number of months up to one year).
Chemical disinfection is an effective way of prevention from and/or suppressing of fish diseases. Usually accessible disinfective preparations are used in fish culture (e.g. burnt lime, chlorinated lime, nitrogen lime, natrium hydroxide, potassium permanganate, formaldehyde, Chloramine, Chlorseptol, Jodonal etc.). Burnt lime is mostly employed for disinfection of the bottom of ponds and reservoirs in the dose of 2.5 – 3 t.ha-1, or chlorinated lime in the dose of 0.5 – 0.6 t.ha-1. In case of myxosporoses, nitrogen lime (5 t.ha-1, or 0.5 kg.m-2) is to be applied. Immediately after fishing out the pond, the disinfection of fishing pit, pond ditches and muddy wet places is performed on large ponds where the whole-surface bottom disinfection is not possible. 5% water solution of formaldehyde, chlorinated lime (200 – 400 mg.1-1), 0.5 % water solution of natrium hydroxide, Chloramine and chlorseptol (30 g.1-1) or other disinfectants can be used for treatment of concrete channels, troughs and other arrangements employed for fish culture. The same disinfectants and concentrations are to be used for the treatment of the equipment. Potassium permanganate (5 g.l-1), Jodonal (2.8 – 4.5 ml.l-1) and other disinfectants can be also employed for these purposes.
d) Optimalization of environmental conditions
The optimalization of natural environmental conditions is the main pre-condition how to ensure the good health condition of stock during the rearing period. The following principles must be ensured:
e) Regular control of health condition and preventive treatment of fish
Preventive control of health condition is to be carried out in hatcheries and early fry rearing units twice a week, and in highly productive intensificated ponds, trout farms and fish culture units with recycling warmed water weekly. Other stocks (esp. in usual pond culture) are investigated monthly.
Health condition of fish is always to be controlled before fishing out, transporting fish and stocking. Preventive treatment can be suggested on the base of investigational results. This treatment is performed above all by the application of medicaments into the water environment and feeding by medicated feeds. More detailed principles of this type of treatment are presented in Chapter 2.2.2.
f) Other preventive principles
The ways of prevention from individual, most important viral, bacterial, fungal and parasitic diseases are described in adequate individual chapters.
Specific, very effective way of prevention from diseases is the vaccination of fish. Vaccines against following relevant viral and bacterial diseases are recently tested with different success: CCV, IPN, SVC, VHS, IHN, furunculosis, ERM, and vibriosis. Individual vaccines are applied intraperitoneally, perorally or in the form of bath. Peroral application or bath are most suitable ways from the point of view of fish culture practice. Also vaccines against some other fish diseases including parasitoses are currently developed.
Recommended literature
Bauer O.N., Musselius V.A., Strelkov Ju.A. (1981): Diseases of pond fish. Izd. Legkaja i piščevaja promyšlennost, Moskva, pp. 318 (In Russian).
Ellis A.E. (1988): Fish vaccination. London, Academic Press, pp. 255.
Lucký Z. (1978): Veterinary care in fish culture. ÚVO, Pardubice, pp. 205 (In Czech).
Prost M. (1989): Fish diseases. Warszawa, PWRiL, pp. 460 (In Polish).
Reichenbach-Klinke H.H. (1980): Krankheiten und Schãdigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, pp. 472.
Roberts R.J. (1989): Fish pathology. Ballière Tindall, pp. 467.
Schãperclaus W. et al. (1979): Fischkrankheiten. Akademie-Verlag, Berlin, pp. 1089.
Svobodová Z., Zajíček J. (1989): Control of fish protozoases. In: Lom J., Dyková I.: Protozoal parasites of important fishes. SZN, Praha, pp. 102 (In Czech).
(Z. Svobodová)
Fish are subjected to therapy in those cases when a disease is so developed that the life or performance of the fish is immediately endangered or expected to be endangered in the subsequent period. Therapeutic treatment should be regarded as emergency measure resorted to when prevention has failed.
The therapeutic treatments may be as follows:
Application of therapeutic substances and preparations to the aquatic environment (therapeutic baths for fish and eggs)
Therapeutic substances are put into water to control ectoparasitic, fungal and bacterial diseases of the body surface and the gills. In some cases the therapeutic baths can also be used (after absorption of the active substances via the skin) for controlling the causative agents of internal diseases.
According to the lenght of exposure, the therapeutic baths are subdivided as follows:
The long-term baths also include the treatment, with therapeutic substances, of whole fish culture reservoirs and ponds. A list of preparations and substances most frequently used for the different types of baths is given in Table 7.
Table 7: Chemical substances used for therapeutic baths of fish
| Type of therapeutic bath | ||
| immersion | short-term | long-term |
| lysol | NaCl | malachite green |
| lime milk | formaldehyde | trichlorphon |
| KMnO4 | malachite green | acriflavin |
| ammonia and | malachite green | antibiotics |
| trypaflavin | and formaldehyde | Metronidazol |
| malachite green | NaCl | |
| CuSO4.5H2O | KMnO4 | formaldehyde KMnO4 |
General principles of therapeutic baths for fish
To perform the therapeutic baths effectively and to avoid losses of the fish, a number of general principles must be respected, including:
a) The state of health of the fish stock must be continuously monitored so that the most effective therapeutic bath can be promptly chosen and applied: fish in an advanced phase of a disease are exhausted and weak and can be easily killed by exposure to the drug in the bath.
b) The results of examination of the fish serve as a basis for determining the type of therapeutic bath. Most of the therapeutic preparations are toxic to the fish at higher concentrations, so the instructions have to be strictly adhered to. The substances and preparations used for the baths must be fresh, packed in original containers. The dose to be used in the bath must be accurately calculated to avoid poisoning the fish by overdosage, or to avoid a poor effect if the dose is too low. If the instructions state a range of doses between two limits, then the lower amount is given to the weakened fish and the higher one to fish in good condition. The drugs must have been dissolved before application to the water; the application itself is performed by spraying over the water surface. With the substances and preparations used for long-term therapeutic treatment of fish in reservoirs and ponds, there should be a satisfactory difference between the lethal concentration (LC) for the causative agent of the disease and the LC for the fish: the therapeutic index* is to be at least 4 or above 4, 10 at the maximum. These therapeutic means must be readily soluble in water and must easily break down.
c) Fresh and uncontaminated water must be used to prepare the solution for the bath. The physico-chemical characteristics of the water influence the effectiveness of the therapeutic substances and preparations and also their toxicity to the fish. The most important water characteristics include temperature, pH, concentration of organic substances, acid capacity (alkalinity), ∑ Ca + Mg and others.
d) A tolerance test must have been conducted before any bath. The tolerance test is a bioassay on several fish to see the safety or harmfulness of the therapeutic bath for the fish stock to be treated under the existing conditions.
e) The therapeutic baths themselves are carried out in all-glass tanks, fibre-glass tubs, vats, fibre-glass plastic troughs, in concrete or earth storage basins or straight in the ponds. It is also possible to subject the fish to short-term therapeutic baths in the transport boxes during shipment if the shipment time is the same as, or shorter than, the recommended exposure time.
The fish should have been given no feed before an immersion bath or a short-term bath to avoid increased need for oxygen (for example, one to three feedings are skipped on the trout farms). Fish exposed to long-term baths, with several days' exposure times, have to be fed with supplementary feeds. Emergency scenarios must be prepared for the prevention of possible accidents: water aeration facilities must be ready for use, or precautions should be made for promptly removing the fish from the bath and putting them in fresh (preferably flowing) water, or an emergency inlet of clean and safe water must be available for fast dilution of the bath solution. The tanks or reservoirs with the therapeutic solutions should never be overstocked: the fish must have enough space to move freely and the solution must get to every spot on the body surface of each fish. A 100-litre bath will accommodate 30 kg of fish at the maximum and the bath solution is as a rule replaced after treating 5–10 sets of fish.
For long-term baths straight in the pond, the substance or preparation is either applied in a single batch into the inlet or may be evenly distributed over the water surface in the pond. For the whole period of treatment the flow of water through the pond must be stopped and warning plates should be placed around it. Residues of the therapeutic substance must have completely disappeared before water is allowed to flow through the pond again. Treatment of the whole pond is seldom resorted to: it is carried out when the fish are in acute danger. It is a problem with such large scale baths that together with the causative agents of the disease the drug used in the bath also kills the organisms in the food chain, thus reducing the nourishing capacity of the pond.
f) When the treatment is finished the fish should be removed from the bath and put into clean (preferably flowing) water. If the treatment was performed in a whole pond, the inlet source must be strong enough to allow for rapid dilution of the bath solution. All regulations and standards regarding surface water quality conservation must be respected in discharging the used therapeutic solution outside the fish culture facility. In the majority of cases the used solutions are disposed of outside the aquatic environment: for example, they are left to seep into the ground in places free of the danger of penetration into surface or underground waters.
g) The effectiveness of the therapeutic baths must be checked by macro- and microscopic examination of 5 fish at the minimum from each pond or tank after the rinsing of the treated fish in clean water. This must be done immediately after the bath, within one day of the termination of the bath at the latest.
h) It is a general principle that market fish should not be treated by therapeutic baths 14 days before shipment to the market. Treatment of market fish in malachite green bath must be avoided for 6 months before assumed time of consumption.
i) All labour safety precautions must be taken during the treatment of fish by therapeutic baths.
A survey of the most important chemicals and preparations used in the therapeutic baths of fish. Preparing and performing the baths
Sodium chloride (NaCl) is widely used in fish culture for parasite control during the rearing of the fish from the earliest stages of the fry up to the market fish. As the difference between the lethal concentrations of sodium chloride to fish and parasites is not very large, it is necessary during the treatment to stick to the general principles, especially the instructions concerning the tolerance tests. Zinc-coated containers should never be used for the NaCl baths. Sodium chloride is largely used in the form of short-term baths which are fairly effective in the control of the species of the genera Cryptobia, Ichthyobodo, Chilodonella, Trichodina and Trichodinella, and somewhat less effective in the control of the species of the genera Dactylogyrus, Gyrodactylus, Piscicola, Argulus, and in the cases of the fungal diseases.
The salt bath is prepared by dissolving 10 to 30 g NaCl in one litre of water. The exposure time is 15 to 30 minutes. If necessary, the salt bath may be repeated in the majority of species. In the early stages of the fry, treated at a water temperature of 20 to 25°C, good results are obtained at a concentration of 10 g per litre and at an exposure time of 30 minutes. In cyprinid culture, NaCl concentration of 20 g per litre is used at an exposure time of 15 minutes for the treatment of weaker fry. Baths of the same characteristics may also be used for the treatment of salmonids. Stronger fry and older fish of the cyprinid group may be treated with success by a bath at a concentration of 30 g per litre for 25 to 30 minutes. It should be taken into account that at water temperatures below 5°C the effectiveness of the salt baths is substantially reduced. Sodium chloride may also be used for long-term baths (concentration of 1–2 g per litre, exposure time 1–2 days) in cases of occurrence of chilodonellosis in fish kept in storage ponds or provisional handling ponds in autumn or spring.
Formaldehyde is distributed in the form of 36–38% aquatic solution. The chemical to be used for parasite control in the fish must be a clear solution free of paraformaldehyde sediment (white sediment on the bottom). During the bath itself, the main factor to be taken into account (among the factors underlying the effectiveness and toxicity of the bath) is water temperature. The market fish may be treated with formaldehyde bath 14 days before delivery to the market at the latest. Formaldehyde is largely used for the short-term baths to control pests of the genera Cryptobia, Ichthyobodo, Chilodonella, Trichodina, Trichodinella, Dactylogyrus, Gyrodactylus, and the fungal diseases.
The concentration of formaldehyde in the bath depends on water temperature. At water temperatures up to 10°C the concentration is 0.25 ml of 36–38 % aqueous solution per litre, at 10–15°C it is 0.20 ml per litre, and at a temperature above 15°C it is 0.17 ml per litre. The time of exposure is 30 to 60 minutes. For example, a concentration of 0.25 ml per litre and exposure time of 30 minutes at a water temperature of 25°C are recommended for the treatment of the early fry stages of cyprinids and catfish.
Long-exposure formaldehyde baths can be used in the same cases of long-exposure NaCl baths, the concentration of formaldehyde (36–38% aqueous solution) being 0.025–0.030 ml per litre. The solution is unrepeatedly applied to the water inlet and there is no time limit of exposure.
Malachite green is deep green in colour, readily soluble in water. Exposure to a therapeutic malachite green bath without prior tolerance test may kill a whole stock. Hence, every new batch of the chemical must be tested for toxicity to fish and effectiveness of parasite control before it is used for the treatment. Some limits apply to the use of malachite green in fish culture: for example, it should not be used for the treatment of the fish later than 6 month to delivery to the market (the hygiene aspect) and at the recommended concentrations and exposure times it should not be used for the treatment of the early stages of the fry (the fish safety aspect).
Malachite green is used either for the short-exposure baths or, more frequently, for long-exposure baths, especially for the control of Ichthyophthirius multifiliis and also in the cases of occurrence of the species of the genera Cryptobia, Ichthyobodo, Trichodina, Trichodinella, Chilodonella and for treatment of fish against the fungal diseases. To control the fungal diseases, it is also possible to use immersion baths in malachite green (66.7 mg per litre, exposure for 10 to 30 seconds). Recently very good results have been recorded with the use of a combined malachite green and formaldehyde bath (exposure for 2 or 6 hours).
The short-term malachite green bath uses a concentration of 6.7 mg per litre and an exposure time of 1 to 1.5 hours. At water temperatures of up to 10°C it can be performed in different types of reservoirs but when the temperature is higher it can only be done in ponds or tanks that can be drained and filled again in 30 minutes.
For the long-term malachite green bath of cyprinids, the chemical is applied at a concentration of 0.5 mg per ml, after accurate calculation of the amount of water in the reservoir or tank. For salmonids the concentration is 0.15 to 0.20 mg per 1. Upon the application of malachite green and its thorough distribution throughout the tank, the water flow is stopped and aeration is provided. Twenty-four hours later the bath is replaced: the tank is drained, clean water is left to flow through it for an hour, the tank is filled again to the same level as before and another dose of the chemical is applied. All this is done six times.
For the combined malachite green and formaldehyde bath, the water in the tank or pond should contain 0.25 mg malachite green and 0.125 ml of 36–38 % aqueous solution of formaldehyde per litre. In fibre-glass troughs the exposure time is 2 hours (with aeration provided) and in the storage ponds 6 hours. In practice this is done as follows: the pond is drained to contain half as much water as normally, the flow is adjusted to a rate at which the water is replaced in 6 hours, and the calculated amount of solution is slowly added to the water inlet. Six hours later the flow is increased to speed up the diluting process and to increase the amount of water in the tank or pond to the normal level. When fish are treated for ichthyophpthiriasis in laminated plastic troughs, it is recommended to repeat the combined bath twice or three times in one week.
Substances and preparations containing copper are used for the therapeutic baths of fish, though they have a toxic action in the aquatic environment. CuSO4.5H2O is used most frequently for the control of some fungal, parasitic and bacterial diseases of fish. At the present time its use is limited to the control of flexibacteriosis of the gills in the salmonids. It is used in the form of immersion bath (concentration 0.5 g per litre, exposure for 1 min) and good results are also obtained when the chemical is applied to a flow-through tank.
Another substance used for the parasite-control treatment of fish is copper in the form of oxychloride [3Cu(OH)2.CuCl2.H2O]. It is used for short-term baths when the fish are found to harbour species of the genera Cryptobia, Trichodina, Trichodinella and Chilodonella. This substance is also a good molluscocide, used to control aquatic molluscs, especially those of the genus Lymnea, which are intermediate hosts of the causative agent of serious fish parasitoses. Preparations based on copper oxychloride include Kuprikol 50, which contains 47.5 % of the active ingredient at the minimum. It is used at a concentration of 30–70 mg per litre for 15–30 min for the treatment of common carp and grass carp. With other fishes the therapeutic dose of Kuprikol 50 is at the level of lethal concentrations. To kill the water molluscs, Kuprikol 50 is used at a rate of 15–30 kg per ha (if the average depth of the pond is 1 m).
The therapeutic efficiency of CuSO4.5H2O and copper oxychloride, as well as their toxicity to fish, is significantly influenced by the physical and chemical properties of the water.
Trichlorphon is used for long-term baths for cyprinids. For salmonids it is very poisonous, so it cannot be used for therapeutic baths in these species. The preparations on trichlorphon basis, used for the baths, are distributed under the brand names Masoten, Neguvon, Dipterex, Soldep and others.
When used for fish parasite control, the trichlorphon-based preparations are applied to ponds or other fish culture facilities (tanks, troughs) at a single dose. The minimum exposure is 48 hours. The parasites on the invaded fish are immobilized during the first day of treatment. In 24 hours the intensity of invasion is considerably reduced and the percentage of immobilized parasites highly increases, in 48 hours the treatment results in a negative parasitological finding. Preparations on the basis of trichlorphon can only be applied to ponds and other facilities with perfectly tight outlet systems. During the treatment and as long as the residues of trichlorphon and its metabolite dichlorvos remain in the water, the flow through the pond must be stopped and there must be a warning plate on the pond dam. The water flow through the pond may be resumed 2 to 3 days after getting a negative result of the bioassay: the time of persistence of the action of trichlorphon and its metabolites is determined by bioassay on daphnias. The average persistence time of these harmful substances in the pond is 1–2 weeks at a water temperature of about 20°C and water pH of 7–8, and 2–3 months in winter, when the water temperature and the pH are low. Long-continued exposure of pond water to trichlorphon and its metabolite, dichlorvos, kills the majority of the natural food for the fish. Owing to this, full-value feeds must be administered until the natural food organisms develop again in the pond. Two weeks must have elapsed from the day of getting a negative result of the test on daphnias, before the fish may be taken from the treated pond for human consumption. The rates of administration of Soldep, containing about 25 % trichlorphon, can be used as an example. Soldep at a concentration of 1–2.10-3 ml.litre-1, i.e. 10–20 litres per ha at an average pond depth of 1 m, is used to kill the species of the genera Dactylogyrus, Gyrodactylus, Piscicola and Argulus, and is also partly effective in the control of the genus Ergasilus. At the same concentration, Soldep also kills the intermediate hosts (Cyclops, Mesocyclops) of some fish parasites, e.g. the tapeworm Bothriocephalus acheilognathi.
The rates of administration of other organo-phosphorus preparations is proportional to the content of the active ingredient, trichlorphon. The use of trichlorphon-based preparations in ponds must always be well-thought and should only be resorted to when the fish stock is exposed to immediate danger.
Ammonia is used in combination with trypaflavin (acriflavin) in the form of immersion baths to kill the pests of the genera Dactylogyrus, Gyrodactylus and Diplozoon; the same bath may also be used when species of the genera Trichodina, Trichodinella and Chilodonella are found in the fish. The ammonia and trypaflavin baths are prepared from a store solution, which consists of 100 parts of 10 % NH4OH and one part of 2.5 % aqueous solution of trypaflavin. The solution for the bath itself is prepared by diluting the store solution with water at a rate of 1:1000. The time for which the fish are left in the bath depends on water temperature. At temperatures up to 12°C the exposure time is 2.5 min, at temperatures above 12°C (up to 20°C) the fish are treated for only 1.5 min. No baths are performed at temperatures above 20°C. Owing to the toxicity of ammonia to fish at higher water temperatures and water pH, the use of ammonia and trypaflavin baths has been much less frequent in recent years.
Acriflavin (trypaflavin) is a brown-red crystalline powder soluble in water. The recommended therapeutic acriflavin concentrations are several times lower than the lethal concentrations to fish (the therapeutic index is about 5). For this reason, acriflavin baths can be regarded as comparatively safe to fish. Acriflavin is used in the form of long-term baths (concentration of 10 mg per litre, exposure for 10 hours), most commonly in aquarium fish culture: owing to the long exposure time, these baths are not very common in fish farming. Acriflavin controls protozoan parasites of fish and bacterial diseases on the surface of the fish body. German authors recommend to use long-term acriflavin baths at a concentration of 3 mg per litre (exposure time 12 hours-repated three times) for the control of local flexibacterioses in trout, eel and carp.
Lime milk is prepared by dissolving 2 g of newly burnt lime in one litre of water. It is used in the form of immersion baths to kill Piscicola geometra. Carp fry are exposed to this bath for 5 seconds. For cachectic stock fish after poor hibernation the exposure time is 10 seconds and for stock carp in good condition, and for older carp, the exposure time ranges from 15 to 20 seconds. Lime bath is not recommended for fish with sensitive gills (pike, trout).
Lysol is a disinfectant aqueous solution of cresol with potassium soap. It is used at a concentration of 2 ml per litre in the form of immersion baths (5–15 seconds) to control the species of the genera Argulus and Piscicola. Lysol is not recommended for brood fish of salmonids.
Potassium permanganate is used in the form of immersion baths (1 g per litre, 30–45 seconds), short-term baths (0.1g per litre, 5–10 min; 0.01 g per litre, 60–90 min) as well as long-term baths for the control of fungal diseases, parasites (when protozooses occur) and bacterial diseases. A potassium permanganate bath at a concentration of 1 g per litre for 150 second was tested with good results for the control of Eudiplozoon nipponicum in higher age categories of carp. This bath cannot be used at temperatures higher than 10°C. Long-term treatments are performed in storage ponds or other ponds for easy fish handling; the concentration is 0.3 to 0.6 mg KMnO4 per litre of water, exposure time 12 hours. The therapeutic doses of potassium permanganate are very close to the lethal concentrations to fish, so the treatment must be performed very carefully, especially with the aquarium fishes. It should be borne in mind that in summer when the water is warm these baths may be dangerous to fish. When brood fish are handled, local injuries on their bodies are treated with a pledget or sponge soaked with potassium permanganate.
Antibiotics are recommended to be used in the form of therapeutic baths to control bacterial diseases of the skin and gills of fish. These baths are used mainly in aquaristics and today also in rearing young stages of fish in special fish culture facilities. Before the treatment, the antibiotic must be well determined as to its performance in the control of the bacteria responsible for the disease the fish suffer from. The therapeutic doses of antibiotics are in the order of tens of mg per litre at long-term baths and in the order of hundreds of mg at short baths.
Entizol, whose active ingredient is metronidazol, can be used for baths at a concentration of 4 mg per litre for 2–3 days. Metronidazol is absorbed via the gills and produces in the blood a therapeutically effective concentration to kill parasitic Flagellata, e.g. the genus Hexamita. The bath is particularly suitable for the treatment of aquarium fishes.
The method of treatment using a temporary increase in water temperature is performed by successively increasing the temperature of the water with invaded fish to 31–32°C for 3 days and then reducing the temperature again to the starting level. The fish stock gets rid of the infection and acquires an appreciable level of immunity. In fish culture practice this method is used to control ichthyophthiriasis mainly in aquarium fishes and in special warm-water fish facilities. In the rearing of the early stages of cyprinids and catfish, warming is the only efficient and practically applicable method of ichthyophthiriasis control.
Therapeutic baths of the eggs
Malachite green, formaldehyde and sodium chloride are most frequently used in fish culture practice for the control of the fungal and bacterial diseases of fish eggs. Malachite green bath provides a good treatment of the eggs of carp, tench, sheatfish, pike, whitefish and salmonids; its concentrations range between about 5 and 10 mg per litre and exposure times are 5 to 30 minutes once to twice daily. Malachite green is not used for the treatment of the eggs of herbivorous fishes: formaldehyde is better for this purpose, its concentration being 0.05 to 0.35 ml per litre and exposure time 10 minutes once in two hours. Formaldehyde bath can also be used for the treatment of other fishes eggs. Salmonid and whitefish eggs may also be subjected to an immersion bath of sodium chloride at a concentration of 20–50 g per litre. Acriflavin (500 mg per litre, 20–30 minutes) is also recommended for these fishes. Besides these traditional preparations, combined-action iodine-detergent, Jodonal preparations such as e.g. Wescodyne or Incodyne have recently been used on an increasing scale: these preparations control fungi and bacteria as well as the virus diseases of fish eggs.
Administration of therapeutic substances in feed
Administration of drugs contained in feed is now practiced increasingly frequently in all types of fish culture. This approach is advantageous, hence promising, mainly from the point of view of fish farm operation. With cyprinids, the stock must have been attracted and concentrated, as far as possible, around the feeding places, and habituated to the administered feed, before the treatment itself can be started. Administration of the same feed as normally, but containing the drugs, may be performed when there is plenty of oxygen in the water and the fish take the feed greedily. In larger water reservoirs it is difficult to habituate the fish to regular feeding, especially in those reservoirs where a larger amount of natural food is available. With salmonids it is very easy to administer drugs with feeds. Before the treatment it is recommended to skip one feeding to be sure the fish will take the medicated feed as soon and as greedily as possible. The disandvantage is that the diseased fish take successively decreasing amounts of the feed offered to them. Heavily infected or invaded individuals do not take food at all, so the treatment has no effect on them.
The therapeutic drugs are administered either as medicated granulated feeds or are admixed to the feeds straight on the fish farm.
In the medicated granulated feeds the drug is incorporated in the pellets. The pellets are hard but they soften and swell in contact with water, where they remain compact for 12 hours. Four medicated feeds are available in Czechoslovakia at present.
These are:
VR (formerly called Karpex; the 5 kg packages distributed
through pharmacies are called Rupin).
Composition: chloramphenicol palmitate 2.173 g, vitamin A
50 000 i.u., vitamin D3 25 000 i.u., methylene blue 0.3 g,
saccharin 0.05 g, anise oil 0.4 g, stabilizers and obduction
substances 59.68 g, wheat flour added to make 1 kg.
Indication: treatment of carp for erythrodermatitis,
possibly also to control other bacterial diseases in
cyprinids.
Administration: administered in the feeding place at a rate
of 15 g per 1 kg of the weight of the stock per one feeding.
The treatment is repeated 4 to 8 times in an interval of 2
to 3 days, depending on water temperature (two-day intervals
are used when the temperature is above 20°C). The feeding
must always be adjusted so as to let the fish consume the
preparation within 12 hours of administration.
VR-NeO (small packages are labelled Rupin-NeO).
Composition: oxytetracyclin 1.33 g, neomycin 0.67 g,
methylene blue 0.3 g, feed flour to 1 kg.
Indication: infectious diseases of cyprinids caused by
oxytetracyclin- and neomycin-sensitive germs.
Administration: administered in the same way as VR.
Taenifugin carp
Composition: niclosamide piperazine salt 7 g, obduction,
auxiliary and appetizing substances 46.25 g, ground
limestone 100 g, wheat flour to 1 kg.
Indication: bothriocephalosis, caviosis and caryophyleosis
of cyprinids, proteocephalosis of rainbow trout.
Administration: Taenifugin carp is used in any season of the
year when the fish take food. It is administered in the
usual feeding places either once or repeatedly in 48 hours
to allow a maximum number of the fish to take the pellets.
The amount given to the fish should be equal to 1–2 % of the
weight the fish stock has at the time of treatment. The
efficiency of the treatment must be checked by a
parasitological examination of the guts 2–4 days after the
treatment. If live tapeworms are found to occur again, the
treatment should be repeated.
Chronicin salm
Composition: chloramphenicol 30.0 g, potassium propionate
100 g, feed flour to 1 kg.
Indication: furunculosis of salmonids.
Administration: Offer the medicated feed in the place where
the common feed is normally given, do this daily for 7 days.
The amount administered should correspond to 1 % of the
weight of the stock at the time of administration. The
administered dose of about 30 mg per 1 kg of fish weight is
to provide the fish with an effective chloramphenicol level
for 24 hours.
Other drugs administered in feed include, in particular, various antibiotics, sulphonamides, furazolidon, carboneum tetrachloratum, Entizol and others. These are admixed into the feeds just before administration. The best cyprinid feeds to carry the drugs are wheat groats or wheat flour high in gluten; for salmonids the best feed for such purposes is ground spleen or ground beef.
Antibiotics administered in feeds are used for the control of the bacterial diseases of fishes. Chloramfenicol has been the most widely used antibiotic: it has a wide spectrum of action but at the same time a number of adverse side effects, as demonstrated in recent studies. Chloramfenicol is administered at a rate of 40–60 mg per kg of live weight of the fish for 10–14 days. Its use is now declining, owing to the mentioned side effects. The best antibiotic for the treatment of each particular disease should preferably be selected on the basis of the results of antibiotic sensitivity tests in the pathogenic bacteria.
Sulphonamides can also be used with success for the treatment of bacterial diseases of fish, especially furunculosis in salmonids. They are administered in feed at a rate of 0.1–0.25 g per 1 kg of live weight of the fish for 8 days.
Furazolidon has been tested with success in the control of the bacterial diseases (especially furunculosis of salmonids and erythrodermatitis of carp) and parasitic diseases of fishes (particularly hexamitosis of salmonids and partly also coccidiosis in carp). To treat the fish suffering from bacterial diseases, furazolidon is added to the feed at a rate of 0.1–0.2 g per 1 kg of the live weight of the fish and is administered for 8 days. For the control of hexamitosis of trout the rate is 0.5 g per 1 kg of the weight of the feed and the administration is continued for 10–14 days.
Carboneum tetrachloratum (CCl4) is used for the control of the most widespread spiny headed worm, Neoechonorhynchus rutili. Equal parts of CCl4 and paraffin oil are added to dry feed (groats) and the rate of administration (in single treatment) is 0.5 ml CCl4 per 1 kg of live weight.
Entizol (active ingredient metrinidazol) is a good therapeutic preparation to treat hexamitoses at a rate of 0.25 g per 1 kg of feed, the treatment being continued for 3 days.
Administration of therapeutic substances via a probe
This method of drug administration is resorted to in exceptional cases to treat limited numbers of fish, e.g. for the control of bothriocephalosis and caviosis in the brood fish at sites with the occurrence of these diseases, before the brood fish are transported to another area or country. The therapeutic substance, e.g. nitrosamine piperazine salt, is dissolved in semiliquid starch gel, which is prepared by boiling about 60 g of food starch (Solamyl) in 1 litre of water. In cyprinids the drug is administered via a thick-walled elastic hose of plastic material, connected with a syringe. The hose is introduced along the central longitudinal axis of the upper palate. The moment when the hose hits the pharyngeal teeth can be clearly identified (by feeling the mild stroke). At this moment the hose should be inserted, with slight twisting, between the pharyngeal teeth and the crushing plate. The hose should be pushed in very lightly and only to a depth where it opens into the gullet (Fig. 17), and then follows the administration of the drug.
Fig. 17: Administration of drugs via a probe
Administering therapeutic substances by injection
In the past the injection method of administration of therapeutic substances was used on mass mainly in the treatment of stock carp. Intraperitoneal administration (into the body cavity) was used mainly with chloramphenicol and later also with the vaccine against spring viraemia (prevention of the disease). However, mass use of these treatments is now becoming less common because of the great laboriousness and of the frequent mechanical injuries and stresses. The therapeutic and preventive substances are administered in feed, as far as possible.
Nevertheless, injection treatment will continue to be practiced in small groups of fish, especially the brood fish. Brood fish may receive in this way, for example, different antibiotics, vaccines, sexual hormones (in the prespawning period) and other substances; T-globulin injections are used in Poland to increase non-specific resistance of brood fish and their progenies. The drug or sexual hormone is injected into the body cavity (intraperitoneal administration) or into the muscle (intramuscular administration).
For the intraperitoneal injection, the site where the needle is to be injected is on the left side of the fish body at the point of intersection of two fictitious lines, the first starting at the base of the pectoral fin and runing along the longitudinal axis of the body and the other starting at about the centre of the pelvic fin and running perpendicularly to the first one (Fig. 18). The angle at which the needle is introduced into the body is also important. In the scaleless fish it should be 20 to 30 degrees, in scaly fish it should be 10 to 15 degrees, the needle passing between two successive scales (Fig. 18). The drug flows easily from the needle introduced in the body wall, visible blotches occur under the skin.
For the intramuscular administration to the carp, the site of injection is on the left flank 1 to 2 cm behind the fore end of the dorsal fin and 3 to 4 cm below it (Fig. 18). With other fishes the injection site is on the boundary between the first and second third of the body, 2 to 3 cm below the upper line. The needle and the injection site should be wiped with a pledget or sponge, dipped in 1 % solution of potassium permanganate.
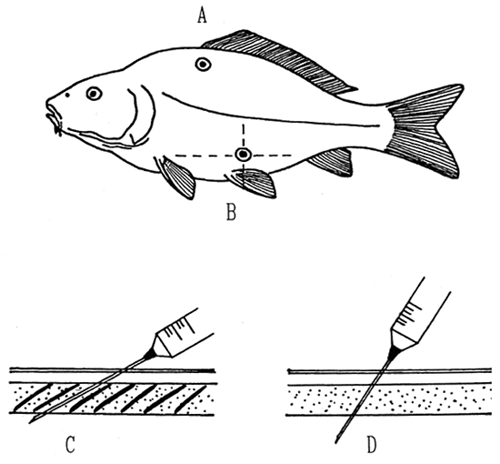
Fig. 18: Administration of drugs by injection. A - Injection site for administration of the drug into the muscle; B - into the body cavity; C - the angle at which the needle is introduced into the body of scaly fish; D - scaleless fish.
Recommended literature
Aldermann D.J. (1985): Malachite green: a review. J. Fish Dis., 8, 289–298.
Herwig N. (1979): Handbook of drugs and chemicals used in the treatment of fish diseases, Charles C. Thomas, Illinois, USA, pp, 272.
Kouřil J. et al. (1991): Antiparasitic and antifungal baths for the early fry of common carp, phytophageous fishes and sheatfish. Research Institute of Fish Culture and Hydrobiology, Vodňany, pp. 8.
Prost M. (1989): Fish diseases. Warszawa, PWRiL, pp. 460 (in Polish).
Reichenbach-Klinke H.H. (1980): Krankheiten und Schãdigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, pp. 472.
Roberts R.J. (ed) (1989): Fish pathology. Ballière Tindall, pp. 467.
Schãperclaus W. et al. (1979): Fischkrankheiten. Academie-Verlag, Berlin, pp. 1089.
Svobodová Z., Faina R. (1989): Application of Soldep preparation in fish culture. Edice Metodik, VÚRH Vodňany, No.12, pp. 15 (In Czech).
Svobodová Z., Faina R., Vykusová B. (1985): Application of Kuprikol 50 preparation in fish culture. Edice Metodik, VÚRH Vodňany, No. 19, pp. 10 (In Czech).
Tesarčík J., Rajchard J. (1983): Veterinary preparations in fish culture. Edice Metodik, VÚRH Vodňany, No. 11, pp. 11 (in Czech).
ON 46 6809 Antiparasitic and antimycotic bath of fish. ÚNM, Praha, 1983, pp. 14 (In Czech).
(J. Tesarčik)
Prevention and therapy are stated for the following major viral diseases of freshwater fishes:
Channel catfish virus (CCV)
Prevention efforts are focused on avoiding infection of the rearing facility through strict veterinary controls on the transfers of brood material. The eggs must come from infection-free environments. Eggs at the eye point stage are preventively subjected to Jodonal bath (concentration of 2.8 – 6.7 ml per 1 litre, exposure for 5 minutes) or to a Wescodyn R bath (concentration 50 mg per 1 litre, exposure for 10 minutes). The channel catfish fry are closely checked for health mainly during the early stage of their life until they reach a weight of 10 g. When an infection breaks out, the infected stock must be isolated, subjected to laboratory examination and - in the event of mass death - safely disposed of. Keeping the fish at a water temperature below 19°C for 24 hours may result in substantial reduction of losses. Upon mechanical cleaning, the infected environment must be disinfected with chlorinated lime (concentration of 200 – 400 ml per litre, exposure for 12 hours). All stress situations are to be avoided. Fish vaccination provides specific prevention of CCV.
No therapy has been developed as yet. Secondary bacterial infections can be controlled by trypaflavine, chlorampfenicol or oxytetracycline baths.
Infectious pancreatic necrosis (IPN)
Prevention relies on strict veterinary controls imposed on all transfers of eggs and fish to uncontaminated fish culture facilities and the flowing waters that feed the rearing ponds. The young fry are best reared from the own stock of each farm and best kept in spring water. The start of intensive fish feeding is a critical period: the fish have to be carefully habituated with no abrupt switchover. The preventative practices also include the general principles of hygiene and disinfection. The regular virological examination of individual trout farms is important for prevention from this disease. Fingerlings at the age of 2–4 weeks and 8–10 weeks is to subject to the examination. The possibilty of this virus registration is the highest in these periods. Fish vaccination provides specific prevention of IPN.
No therapy at a significant efficiency level has been developed as yet. The mortality of the affected stock can be reduced by adding polyvinylpyrrolidone iodine to the feed pellets at a dose of 1.6 – 1.9 g per 1 kg of the live weight of the fish per day for 15 days. This treatment can only be effective if started on time.
Spring viraemia of carp (SVC)
Virus of SVC is spread in all fish culture facilities in our country. Prevention is to be tended to enhance the non-specific resistance of fish. Fish must be reared by well-tested methods, stress should be excluded, fish stock should be optimalized, plenty of natural food and quality replacer pellets should be provided and the fish being reared must be kept under strict health control. It should be combined with preventative antibiotic and chemotherapeutic treatment of the fish and with the control of the causative agent through the disinfection of the fishing gears, transport containers and small reservoirs (storage ponds, wintering tanks) and waterlogged places. Ponds, reservoirs and tanks are disinfected by means of burnt lime applied at a rate of 2.0 to 2.5 tonnes per ha. Apparently diseased fish should be eliminated and suspected stocks should be reared in small ponds away from the rest of the fish farm's stock.
Fish vaccination provides specific prevention of SVC. A vaccine has been developed in Czechoslovakia; it was administered intraperitoneally on an experimental scale. From the point of view of fish farming practice, the most promising method would be the administration of the vaccine in food; this method is being currently finished in Czechoslovakia.
There is no therapy to kill Rhabdovirus carpio. Antibiotics (chloramphenicol, neomycine) or chemotherapeutics (sulphonamides), all administered in feed, are used for the prevention of secondary bacterial infections. Medicated VR or VR-Neo feeds can also be used with advantage.
Viral haemorrhagic septicaemia (VHS)
Prevention relies on preventing the introduction of the causative agents into the fish culture facilities or to the watershed; this is done through strict veterinary controls on any transfers of eggs and stock fish. As to the general preventive measures, emphasis is laid (mainly in intensive culture facilities) on feed quality, copious supply of vitamins, hygiene of the environment and regular veterinary checkups. It is recommended to treat the eggs by Thiomersal, Merthiolat and Bactosept baths (concentration of 0.2 mg) per litre, exposure for 10 minutes). Burnt lime (1 kg per m2), calcium cyanamide (1 kg per m2), chlorinated lime (2 kg per m2) or sodium hydroxide (2 g per litre) can be used for the disinfection of the rearing facilities and the appliances used. Fish vaccination is a specific prevention of VHS.
There is no known effective therapeutic means to control the disease. In is recommended to give the fish easily digestible feed (spleen) enriched with vitamins A, B1, B2, D and E during the critical period. Good response is also gained when the fish are given the Meso-Inosit vitamin mix at a rate of 350 mg per 1 kg of feed pellets for several days at a reduced water temperature.
Infectious haematopoetic necrosis (IHN)
It is possible to provide prevention of the disease by applying the common measures normally taken in cases of any infection. The only effective measure is to keep the stocks away from the causative agent of the disease. It is recommended to use the following practice to prevent the disease: rear the fry at a temperature of 15°C at the minimum for 1 month after hatching; then transfer the fry to normal conditions with a temperature of about 10°C. As to the eggs, it is useful to subject them to regular Jodonal baths (concentration of 4.27 ml per litre, exposure time 10 minutes) or to Wescodyn R bath (concentration of 76 mg per ml, exposure for 10 minutes). Fish vaccination provides specific IHN prevention.
No therapeutic method with a specific IHN control activity is currently available.
Pike fry rhabdovirus (PFR)
Prevention is based on adherence to hygienic principles and implementation of infection control measures; this includes, in particular, the care of the cleanness of all gears and tools and fish culture facilities; it is also necessary to prevent water from dubious sources from entering into the fish culture environment. For prevention it is recommended to dip the eggs in the eye point stage in a Jodonal solution at a concentration of 2.85 ml per litre for 10 minutes or in Wescodyn R at a concentration of 50 mg per litre for 10 minutes.
Swim bladder inflammation
Prevention is focused on timely identification of the foci of the infection and on adherence to the veterinary regulations (the same as for SVC). As to the general preventive measures, plenty of fresh natural food and replacer feeds should be provided mainly to carp fry. Burnt lime can be used at a dose of 2.5 tonnes per hectare for the disinfection of the fish rearing environment.
No specific-action therapy has yet been developed. Secondary bacterial infections which complicate the cases can be controlled by timely administration of antibiotics or chemotherapeutics. The therapeutic intervention can only alleviate the course of the infection and reduce the mortality of the fish. Medicated VR or VR-Neo feeds can be used with advantage, both therapeutically and preventively.
Pox of carp
Prevention relies on the elimination of the factors underlying the occurrence of the disease. When selecting the brood fish the individuals with blisters on the body are eliminated. Lime is applied if the water in the pond or in the feed source is acid. Ectoparasites, especially the fish lice, are strictly controlled.
Indirect treatment can be performed in the pond stocked with diseased fish: apply 50 kg of burnt lime per 1 ha several (three to four) times with an interval of three to four days between each two treatments. Give plenty of feed enriched with vitamins, minerals and trace elements, in case also with chloramphenicol to the diseased fish.
Papillomatosis of eel
Prevention relies on the veterinary checking of the eels during the first and second year after stocking (this can only be done in fishponds); if positive cases are reported, the import of elvers from the infected areas should be reduced. The import of elvers from localities free of this disease is the basic preventative pressuposition.
In practice, as a rule, no preventative measures are taken. The fish showing symptoms of the disease are eliminated from breeding and the infected individuals are killed and burnt. A description has been published concerning the treatment of fish infected with this disease, using quinine sulphate at a concentration of 60 mg per litre for several hours (quinine sulphate bath).
Recommended literature
Ahne W. (ed) (1980): Fish diseases. Spring Verlag, Berlin, Heidelberg, New York, pp. 252.
Ahne W. (1985): Virusinfektionen bei Fischen: Ãtiologie, Diagnose and Bekampfung. Zentbl. Vetmed., B, 32, 237–264.
Ellis A.E. (1988): Fish vaccination. London, Academic Press, pp. 255.
Lucký Z. (1986): Diseases of important fishes. SPN, Praha, pp. 201 (In Czech).
Pospíšil Z., Tománek J. et al. (1991): Diagnosis and prevention of infections pancreatic necrosis in salmonids. Research Institute of Fish Culture and Hydrobiology, Vodňany, Czechoslovakia, pp. 15.
Prost M. (1989): Fish diseases. Warszawa, PWRil, pp. 460 (In Polish).
Reichenbach-Klinke H.H. (1980): Krankheiten und Schädigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, pp. 472.
(J. Tesarčík)
Prevention and therapy are stated for the following major bacterial diseases of freshwater fishes:
Furunculosis
Prevention in flowing waters relies on water purity and sufficient oxygen concentration. Organic pollution of the trout and grayling waters must be as low as possible. Watersheds exposed to the danger of occurrence of furunculosis should be stocked with species less susceptible to the infection (rainbow trout) or with salmonids at a younger age (fry), which are slightly more resistant. Under such circumstances, the number of fry (fish) for the stocking is usually reduced, sometimes even to half the normal level. If the disease breaks out, the dead fish are taken out of the water and safely disposed of (burnt or burried together with a powerful dissinfectant-chlorinated lime or burnt lime). In salmonid culture, the prevention relies on protecting the stock against the introduction of the disease with fish transferred from infected flowing waters or from fish farms where the disease occurs. Immediately after fertilization, or at the eye-point stage, the eggs should be treated with an acriflavine solution at a concentration of 0.5 g per litre for 20 minutes. The preventative measures recommended for flowing waters must be implemented even more strictly in salmonid rearing operations where the intensity of the culture is much higher. Fish vaccination provides specific prevention of furunculosis.
Drugs to control the disease are administered either for prevention or for therapy; in both cases they are added to the food (ground spleen - admixed before administration; pellets - medication during the manufacturing process). These drugs may include powerful antibiotics, sulphonamides, furazolidone; the medicated feed Chronicin salm is also suitable. Burnt lime (dose of 2.5 to 3.0 tonnes per ha) is used for the disinfection of the rearing facilities (small ponds). Fishing gears and tools are disinfected with a potassium permanganate (KMNO4) solution at a concentration of 10 g per litre.
Carp erythrodermatitis (CE)
As to the available preventive measures, it is necessary first of all to handle the fish very carefully during fishing to avoid any skin injury. Try also to avoid injury during the transportation of the fish to longer distances and during stocking. Maintain the fish in good condition and in a good state of nourishment above all by support of natural food sources development in ponds. Antibiotics and chemotherapeutics may also be used for prevention.
The causative agent is very sensitive to antibiotics and sulfonamids so mass administration is the preferred route of introducing these substances in the fish body; parenteral treatment and baths are less frequent. Chloramphenicol is the most frequently used antibiotic: it is incorporated in medicated feeds, e.g. the VR brand. Oxytetracycline and neomycine may also be used: these are incorporated in the VR-NeO medicated feed. Burnt lime (2.5 tonnes per ha) or chlorinated lime (500 kg per ha) are used for the disinfection of the fish culture facilities (ponds). A 10 % solution of the lime milk is used for the disinfection of the gears and tools.
Enteric redmouth disease (ERM)
Strict veterinary controls of transferred fish, and strict quarantine, are the most effective preventive measures. Infected fish farms are allowed to sell no other brood material but eggs, which must have been thoroughly treated with iodine preparations (e.g. Wescodyne R bath) at a concentration of 50 mg per litre for 5 – 10 minutes. Literary sources say that this is sufficient exposure to reliably kill the causative agent of the disease. Vaccination provides a specific prevention of enteric redmouth disease: the immobilized vaccine is applied in the form of bath.
ERM therapy is based on the administration of oxytetracycline or on a combination of sulphonamides with chloramphenicol. Thimethoprin combined with sulphadiazine (e.g. the Duon speciality) is considered as the most effective of the recently developed preparations. Fish therapists in some countries outside Czechoslovakia use oxolinic acid and flumequin, which are even more powerful. All these preparations are administered in feed. The problem with the treatment is that it usually has to be repeated.
Vibriosis
Prevention of this disease relies on direct veterinary inspection of transported fish, on strictly implementing hygienic precautions in fish culture, on bacteriological analysis of the feeds prepared from sea fish, and on respecting the general conditions of the control of bacterial diseases, including quarantine, disinfection, safe disposal of diseased fish and others. Vaccination of the fish provides specific prevention of vibriosis.
The preferred therapy is the administration of sulphonamides or furazolidone in feed.
Bacterial kidney disease
Prevention of this disease requires strict quarantine of the fish brought from sources not subjected to fish health control. If eggs are to be transported they must first be bathed in sodium methiolate at a concentration of 0.2 g per litre for 10 minutes. Jodanal can also be used (concentration of 4.3 ml per litre, exposure for 10 minutes). The eggs may be treated in this way either immediately after fertlization or later, just before transportation, when they are at the eye-point stage. Where the disease persists for a long time, sulphonamides are preventively administered in feed.
If the disease occurs, the dead fish, or the whole affected stock, must be safely disposed of (in a rendering plant). Erythromycine, administered in feed at a rate of 100 mg per 1 kg of the fish live weight for 21 days, gives the best results in the treatment of the fish suffering from this disease.
Mycobacteriosis
Prevention is based on good hygiene of fish culture. This involves regular cleaning of the tanks and avoidance of overstocking. Plenty of varied food should be available to the fish to maintain them in good condition. Stress situations (control fishing, transportation) must be minized and long-term weakening of the fish (caused e.g. by inadequate temperatures or physico-chemical properties of water) must be avoided. Suspect individuals should be immediately removed. When fish are purchased and especially when they are imported from a foreign country, the supplier should be required to provide a veterinary certificate proving that the fish came from a disease-free environment. Nevertheless, in spite of the certificate, the purchased fish must spend some time in quarantine.
Medicamentous treatment in advanced stages of mycobacteriosis usually fails to bring longer-lasting results. However, longer exposure to streptomycin baths in an earlier stage of the ailment can stop the disease: add 50 to 300 mg of the drug per 1 litre of water and leave the fish in the bath for 4 to 6 days. In cases of mass infection it is recommended to remove and destroy all the fish stock and the plants and disinfect the tank and everything inside it (preferably using preparations containing active chlorine).
Myxobacteriosis
This is a group of diseases of which the following three are the most serious:
a) Columnarosis
Prevention of this disease involves careful handling of the fish during fishing, grading and transport. Ectoparasite control is another important factor of prevention. In salmonid culture it is useful to reduce water temperature to a level at which the development of the disease is curbed.
Therapy based on baths is most frequently used in salmonids and in aquarium fishes, using the advantage of the fish being kept in small containers. Immersion bath is used for the treatment of salmonids, the fish being dipped in a solution of blue vitriol (CuSO4.5H2O) at a concentration of 0.5 g per litre for 1 minute. Malachite green is also a good chemical for immersion baths (concentration of 66.7 mg per litre, exposure time 10 to 30 seconds). Sulphonamides are administred in feeds to the fish suffering from columnarosis. Longer-term baths are used in aquarium fish culture, e.g. chloramfenicol at a concentration of 60 mg per litre for six days.
b) Cytophagosis
The therapy is about the same as with columnarosis, except for the recommended reduction of water temperature: for the treatment of cytophagosis it is recommended to increase water temperature. An increase in water temperature above 15°C will suffice to arrest the disease. However, this treatment is difficult to provide in salmonids in many cases.
c) Bacterial gill disease
Prevention of this disease relies on maintaining good hygienic and breeding conditions. The food must contain plenty of vitamins of group B. Trout of the critical size have to be reared in clean water (preferably spring water). The size of the stock must be adjusted according to water purity and water inflow rate. All debris and dirt must be removed from the rearing tanks (especially the residues of food and the excrements). A small amount of cod liver oil may be added to the feed replacer if it is too dusty.
Stocks in chronically diseased rearing facilities are preventively treated in critical periods of the year, or soon after the first signs of the disease occur. The treatment rests in short term or dipping (immersion) baths; during the short term bath an aeration device must be used to maintain sufficient oxygen in the water for the fish. Regularly performed baths in substances containig active chlorine (e.g. chloramine in concentration of 20 mg.l-1 for 20 min.). Immersion baths in blue vitriol (CuSO4.5H2O) give good therapeutic results (concentration of 0.5 g per litre, exposure time 1 to 1.5 minutes). Malachite green is another good chemical for short-term bath treatment. Also sulphonamids and chinolone chemotherapeutics respectively can be applied by peroral way.
Recomended literature
Lucký Z. (1986): Diseases of important fishes. SPN, Praha, pp. 201 (in Czech).
Prost M. (1989): Fish diseases. Warszawa, PWRiL, pp. 460 (In Polish).
Reichenbach-Klinke H.H. (1980): Krankheiten und Schädigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, pp. 472.
Roberts R.J. (ed) (1989): Fish pathology. Ballière Tindall, pp. 467.
Schäperclaus W. et al. (1979): Fischkrankheiten. Akademie-Verlag, Berlin, pp. 1089.
Tesarčík J. Rajchard J. (1983): Veterinary preparations in fish culture. Edice Metodik, VÚRH Vodňany, No. 11, pp. 11 (In Czech).
(J. Řehulka)
Oomycetosis (primarily caused by Achlya and Saprolegnia)
The best of the therapeutic substances is malachite green B (oxalate compound), active even at a very low concentration. The amount used for the dipping treatment is 66.7 mg per one litre of water, the exposure time being 10–30 seconds. For a short bath with an exposure time of 1–1.5 hours the concentration is also 6.67 mg of MG per one litre of water. For a long-time bath (6 days), the concentration must be much smaller, 0.2 to 0.5 mg per one litre of water. The effectiveness of the long-term bath depends on water quality. The fish usually have to undergo the bath again three days later. This approach is suitable for the treatment of fish in large storage basins or tanks. For aquarium fishes and salmonids it is recommended to use the lowest active concentration of malachite green, 0.15 mg per litre.
Market fish should be free of malachite green treatment 6 months before going to the market. The eggs of salmonids can be subjected to preventive and therapeutic treatment at a rate of 5 mg MG per one litre of water for an hour twice a week. This is done without handling the eggs, the store solution being left to drop into the water that flows to the tank.
Saprolegniasis of fish can also be controlled by short potassium permanganate baths. The concentration of the chemical in the bath is 0.01 g per 1 litre of water and exposure time is 1 to 1.5 hours.
Branchiomycosis
Preventive treatment is conducted in ponds where branchiomycosis permanently occurs in July and April, when burnt lime powder is applied onto the water surface at a rate of 50–100 kg per hectare. It is recommended in this period to increase the rate of flow through such ponds to improve oxygen content. It is also advisable to avoid putting too much organic matter in the pond through intensive organic manuring and intensive breeding of water fowl. Burnt lime should be used at a rate of 2.5 t per hectare to dissinfect the bottom. If the disease occurs in its acute stage, stop feeding the fish, increase the flow through the pond and treat the water surface with chlorinated lime at a rate of 10–15 kg per ha (at an average pond depth of 1 m) three times a week at the maximum. When the branchiomycosis is over it is recommended to administer medicated feed (with antibiotics) to prevent secondary bacteriosis of the gills.
Ichthyophonosis
No therapy is available as yet. Prevention is based on thorough veterinary inspection of the fish rearing facilities and quality control of the feed on trout farms where sea fish are administered. The preventive sanitation measures include pasteurization of potenntially infected fish flesh. The reservoirs infested with the fungus are disinfected with burnt lime at a rate of 2.5 t per hectare. Aquarium tanks are drained from above and the bottom and walls are dissinfected with potassium permanganate at a rate of 1 g per 100 litres of water for 90 minutes.
Moniliales
In exophialosis prevention, increased attention should be paid to the quality of food especially the tubificids, which are vectors of Exophiala pisciphila to aquarium fishes. The most vulnerable of the aquarium fishes is Hemmigramus pulcher. In ochroconiosis neither prevention nor effective therapy are available yet. In fusariosis prevention and therapy are similar to those in coelomycetosis.
Coelomycetes
Prevention of this disease is based on maintaining the water at an optimum quality. In places where it occurs the fungi must have been killed in the reservoirs or tanks (by disinfection repeated twice or three times with 20-day intervals between the treatments) before stocking and during the rearing. The disinfectant suitable for the treatment of tanks without fish include a solution of sodium hypochloride with a 2 % content of active chlorine, or a 2 % formaldehyde solution, the exposure time being 20–30 minutes.
Recommended literature
Amlacher E. (1986) : Taaschenbuch der Fischkrankheiten. VEB Gustav Fischer Verlag Jena.
Neish G.A., Hughes G.C. (1990): Fungal diseases of fishes. T.F.H. Publications, Inc., Ltd.
Wolke R.E. (1975): Pathology of bacterial and fungal diseases affecting fish. In: The Pathology of Fishes (ed by W.E. Ribelin and G. Mikagi), 33–116. The University of Wisconsin Press, Madison, Wisconsin.
(Z. Svobodová)
The methods of prevention and therapy of important parasitic diseases are described as follows.
Protozooses
1. Fish diseases caused by ectoparasitic Protozoa
Ichthyobodosis
Prevention of ichthyobodosis should be realized with absolutely full care. The main effective rules of prevention are:
a) To use the source of water free of parasites and their cysts.
The underground water is the most suitable source of water free of parasites. Nevertheless, these sources are very limited even for trout farms, hatcheries and/or other special fish culture facilities. In most cases, water from streams is used as a source of inlet water. In such cases, suitable filters can free the inlet water from infectious stages of parasites prior the water enters smaller tanks with intensive fish culture. A water from ponds stocked with fish is absolutely unsuitable to be used in trout farms, hatcheries and/or rearing facilities with early stages of fishes.
b) The stocking of healthy fish
Only well examined fish, free of ichthyobodosis and treated by antiparasitic bath (in case of necessity) should be stocked into ponds and fish rearing facilities.
c) Creation of presuppositions for good performance and health conditions of fish.
It refers particularly to the optimum quality of water without physical and chemical stressing effects. It is necessary to avoid the stress effects caused by other factors too, namely by inconsiderate handling with fish. Through appropriate culture management, the maximum development of natural food should be gained. The supplementary feed must be applied in sufficient quantity and quality. Abundant natural food of suitable species and size composition is a basic preventive measure in rearing the early stages of fry. The maximum level of stock density must be calculated carefully. Unreasonably dense stock in trout ponds and/or raceways, in fry reared in special culture facilities, in any other fish production units, and in ponds too, leads to stressing situations, worsens the nutritional state of fish, decreases the resistance and opens the door to infections.
d) Regular control of health condition of fish
A preventive control of health condition of fish reared in hatcheries and in special fish culture facilities (in case of early fry rearing) must be realized twice a week, in trout farms once a week. A control of health condition must be preformed just prior the fishing, the fish transportation and/or stocking the fish.
Therapy - Many antiparasitic baths and their modifications have been proposed as a therapeutic measure. The effectiveness of baths is not absolute; some flagellata survive in folds of epithelium which creates new presupposition for infection. Basicaly, short-term baths in sodium chloride and/or formaldehyde are used for ichthyobodosis control treatment in early stages of cyprinids and European catfish, reared in facilities utilizing the heated effluents. In salmonid fish culture, a long-term bath in malachite green is recommended in most cases from practical aspects. In cyprinids and salmonids, a two-hour- and/or six-hour-long combined bath in malachite green and formaldehyde can be used too. A long-term bath in acriflavine is recommended in aquaria fish culture for ichthyobodosis control.
Cryptobiosis
Abundant natural food of suitable species and size composition preventing the condition impairing of fish is a basic preventive measure in rearing the early stages of fry and preventing the protozoal infection. Optimum quality of water is an another important aspect. In case of cryptobiosis, a frequent sludge removing from rearing tanks is a necessary measure preventing the increase in organic substances concentration and level of organic detritus in water.
To control the dense growths of Cryptobia on the body and the gill surface, a decrease of organic load and of organic detritus (by frequent sludge removing from rearing tanks) together with improving the quality of nutrition (by abundant natural food) is mostly sufficient. In case this measurement is not effective, the same baths as in ichthyobodosis can be applied.
Ichthyophthiriosis
Prevention is based both on the stocking of healthy fish and the use of water free from parasite infectious stages. In special fish culture facilities with heated water, in trout culture, and in special wintering ponds, inlet water from lower layers and/or from retention reservoirs without fish stock should be used. In case of inlet water from streams, water must be filtered through gravel-sand filters. If diseased fish have appeared in fish culture facilities or ponds, tanks and pond bottom must be completely dried after fishing and disinfected with calcium oxide (2.5 t.ha-1) and/or calcium hypochlorite (0.5 – 0.6 t.ha-1). The fishing equipment contacted with water and/or fish from infected environment, must be thoroughly dried. It is important to take into mind when controlling ichthyophthiriosis, the encysted parasites (tomonts) can survive in aquatic environment 1 week in water temperature 20°C and 2 weeks in low temperatures. After this period, aquatic environment is free from parasites. The creation of optimum living conditions is another important presupposition ensuring the good health state of stocks in course of their culture. In case of ichthyophthiriosis prevention, a respective good nutritive and health state of fish is a presupposition together with appropriate density of stock, particularly in early fry rearing and in wintering ponds. Unreasonable dense stock enables the spreading of infection. When fish are kept in good condition, parasites survive in minimum numbers. In weakened fish, in decrease of their resistance and/or in increase of water temperature (which is common in spring period in wintering ponds), parasites reproduce intensively.
Therapy of ichthyophthiriosis is difficult. Due to immersing into the surface tissues of the fish body, parasites are more or less protected against the antiparasitic baths. A method of temporary increase of water temperature and antiparasitic bath can be used in therapeutic treatment of ichthyophthiriosis. Method of temporary increase of water temperature to 31 – 32°C for 3 days is well proved in aquaria fish culture and in special fish culture facilities utilizing the heated water. Antiparasitic baths must be used in period when adult trophonts leave the surface of host to produce a cyst. Baths are effective to these free stages of parasite only. Most frequently, long-term bath in malachite green is applied. Based on specific culture conditions, short-term baths in malachite green can be used too. Recently, a repeated combined bath in malachite green and formaldehyde for 2 hours has been proved with success in aquaria and troughs, and for 6 hours in earth ponds and holding ponds.
Chilodonellosis
Prevention of chilodonellosis is based in creating the optimum conditions of fish culture and in preventing the transfer of causative parasite. To prevent the infestation of rearing tanks, the diseased fish are not allowed to stock and gravel-sand filters are recommended to install on inlets to special fish culture facilities. Optimum quality of water is important as well; low content of organic substances and high content of oxygen in water is unfavourable for parasite. An appropriate nutrition of fish, particularly prior the wintering, is an important preventive measure.
Therapy of chilodonellosis is realized through several antiparasitic baths which, unfortunately, are not very effective in certain cases. Short-term baths in sodium chloride, formaldehyde, malachite green and bath in Kuprikol 50 as regards to carp and grass carp can be mentioned. As to long-term baths suitable for chilodonellosis control, bath in malachite green (fish kept in holding ponds) and long-term bath in sodium chloride and formaldehyde can be applied. A long-term bath in acriflavine is used in aquaria fish culture.
Trichodinosis
Prevention is identical with measure for skin and gill protozooses.
Therapy is identical with chilodonellosis.
2. Fish Diseases Caused by Endoparasitic Protozoa
Trypanoplasmosis, trypanosomosis
Prevention is based in diminishing and control of leeches occurrence. A good condition of fish prior the wintering is important to diminish the losses caused by blood flagellata.
Effective therapy has not been worked out yet.
Hexamitosis
Prevention - To prevent the transfer of cysts into the fish culture facility via both infected fish and inlet water is one of the most important measures. Regular control of health state of fish and microscopic examination of fish excreta (once in a week in case of salmonid fry rearing) is a necessity. In case of positive findings, a preventive dose of furazolidon (0.3 g per 1 kg of feed) is applied daily for 14 days. Appropriate hygienic conditions and sufficient nutrition of fish belong to important preventive measures in hexamitosis control.
Therapy - furazolidon in feed can be used successfuly for therapeutic treatment. The Polish preparation Entizol, applied in feed, is also a very effective drug. This preparation can be used in form of long-term bath as well.
Coccidiosis
Prevention is based on oocysts and sporocysts (occurring on the bottom of ponds and holding ponds) control. Drying the bottom and its disinfection is very effective. Regular wintering, drying and disinfection of muddy parts of nursery ponds with burnt lime (2.5 t.ha-1) and/or chlorinated lime (0.5 – 0.6 t.ha-1) is an effective method of prevention. To prevent the transfer of parasite from older classes of fish and/or from coarse fish to carp fry is an another important element of prevention. In natural spawning of carp, brood fish should be fished out as soon as possible from spawning ponds. In infected cyprinid culture, supplementary feeds must be applied frequently to prevent the fry to feed on benthos due to abundant population of coccidia oocysts.
Therapy - peroral application of furazolidon in dose 0.3 g per 1 kg of feed for 5 days is used in carp fry rearing.
Myxosporeosis
Prevention is focussed on regular veterinary examination of fish and on selection of fish with positive findings out of culture. Most of species of freshwater myxosporea have a very resistant spores, some of them are adapted to temporal drying-up. In natural environment, spores keep their viability for 6 months to 2 years based on different conditions. Soon or later, spores sediment on the bottom, and mud and benthos create a source of infestation. Due to turbulent stream movement, spores are transferred to other localities. Spores can be distributed by fish-consuming birds too (spores were not damaged in bird's digestive tract).
Myxosporeosis control must be focussed on spores as the infectious stage, the only able element in the developmental cycle. Experiments with myxosporeosis therapy failed yet so the spores control is the only measure. Pond bottom disinfection through summer drying and chemical substances application is very important. Optimum method of prevention includes the drainage of pond in the autumn period and application of disinfective substance on the wet bottom. Afterwards, pond is filled with water only to cover the bottom, and disinfective substance is let to act for 3 weeks. Then the pond is flushed-through, drained and dried through the winter period. It is recommended to repeat this process once again in spring time, and then the pond can be filled with water. Calcium cyanamide in dose 0.5 kg per 1 m2 of bottom area is advised as an optimum disinfection.
Therapy of myxosporeosis is not worked out yet.
Myxobolosis
Prevention is focussed on regular veterinary control of fish health state and on control of spores, as it is mentioned in myxosporeosis prevention. Rearing the fry till the age of 2 to 3 months in plastic troughs and/or concrete tanks with inlet of suitable water is another measure in prevention. After this period, fry can be stocked in earth ponds. In ponds permnanently watered from naturally disease-infected localities, rearing the fry till the age of 2 to 3 months is not realized.
Therapy of myxobolosis is not worked out yet.
Metazooses
Monogeneosis
Prevention is based on excluding the parasites transfer from culture environment via both diseased fish and inlet water including the infectious Dactylogyrus and Eudiplozoon larvae. Natural spawning should be restricted and artificial propagation of fish should be utilized which excludes the contact of brood fish and fry. It must be avoided to stock the infected fish into the rearing facilities. Each fish species must be stocked as a single age-class category into ponds. In nursery ponds, inlet water should be free from infected larvae, i.e. source of this water is out of reservoirs stocked with the same fish species. Abundant natural food and supplementary feeding the fry with quality feeds is another preventive measure in carp fry rearing. In such case, 6 cm body length of fry is reached as soon as possible, and in this stage, the fry is relatively resisting to Monogenea (particularly to Dactylogyrus vastator). Till this moment, a regular frequent examination of health state of fish is necessary.
Therapy. Short-term formaldehyde bath in the course of re-fishing the fish, and long-term bath of cyprinids in trichlorphone-based preparations diluted directly in pond, is an effective therapeutic method in dactylogyrosis and gyrodactylosis. It is necessary to note that trichlorphone application in ponds is followed by intensive reduction in natural food abundance. It is therefore necessary to feed the fish intensively with high-quality feeds till the time of re-development of natural food. In case of lower intensity of carp fry infection in nursery ponds, a standard measure to improve the culture environment and health state of fish is applied instead of trichlorphone application. Inlet of oxygen-rich water is increased, rotten vegetation is removed, and fry is fed intensively. Short-term bath in sodium chloride can be mentioned too, nevertheless it is a less used treatment method with no extra effectivity. Ammonia and trypaflavine immersion bath is too risky in conditions of higher temperature and pH of water.
Potassium permanganate bath in concentration 1 g.1-1 for 150 sec is used as therapeutic method in eudiplozoonosis. This bath is recommended for older carp and it is effective up to water temperature 10°C.
Cestodoses
This group of diseases is represented mainly by the following five parasitoses:
Khawiosis
Prevention is based on careful veterinary control of transported fish and fish reared in own culture facilities. The preventive feeding with medicated feed Taenifugin-carp is applied in case of low intesity infection in fry and stock carp. This preventive feeding is obligatory in case of transportation of fish from infected localities to areas free of parasite. As to antiparasitic treatment in brood fish, piperazine salt of niclosamide is applied in starch gel through probe. When khawiosis is registered in growing period, intensive feeding can prevent the fish to consume the tubificids with developmental stages of parasite. Direct control of intermediate host tubificids is not realised in our conditions. Abundance of tubificids is controlled through pond reclamation, winter and/or summer drying of pond bottom, and bottom disinfection (2.5 t.ha-1 of burnt lime or 0.5 – 0.6 t.ha-1 of chlorinated lime). This is also a method to control the occurrence of Khawia sinensis eggs.
Therapy. Single or repeated application of medicated feed Taenifugin-carp is proved as a therapeutic treatment.
Caryophyllaeosis
Prevention is identical as to khawiosis.
Therapy is identical as to khawiosis. It can be applied in pond culture only.
Bothriocephalosis
Prevention is also identical as to khawiosis. A preventive control of intermediate hosts is not realized. Only in extreme mass occurrence of bothriocephalosis, application of trichlorphone-based preparations (e.g. Soldep in dose 10 l.ha-1 for a water column 1 m on average) is used to control the intermediate hosts in a reservoir stocked with fish.
Therapy is identical as to khawiosis.
Proteocephalosis
Prevention is applied in cage culture of rainbow trout only. It is identical with preventive measure in khawiosis and bothriocephalosis. First of all, it refers to careful control of health state of fish, prevention the spreading of parasitosis into other localities, and preventive feeding the rainbow trout with feed medicated with piperazine salt of niclosamide.
Therapy. Single or repeated feeding the trout with feed medicated with piperazine salt of niclosamide in dose 5 g per 1 kg is applied as a therapeutic measure.
Triaenophorosis
Prevention is based on excluding the infected pike from inlet water entering the trout culture.
Therapy is not practised.
Trematodoses
Prevention is based on regular examination of health state of fish. In more intensive infestation, interruption of developmental cycle of parasite is realized by means of control of intermediate hosts. In diplostomosis and postodiplostomosis, occurrence of fish-consuming birds as definite hosts should be restricted in intensive fish culture. This can be done through a control of gull eggs, a use of nets over the trout raceways etc. Snails are controlled by biological, physical and/or chemical method. No any method itself is sufficiently effective. To interrupt the developmental cycle of parasite, individual methods must be combined. In herbivorous fish fry rearing in ponds with intensive occurrence of snails, careful drying and winter freezing of pond bottom is recommended. Afterwards in spring period, a pond is partly filled with water. After leaving the bottom mud, pond snails (Lymnaea stagnalis) are controlled by means of chemicals. The preparations with copper as an active substance (e.g. Kuprikol 50 in dose to 30 kg.ha-1 for a water column 1 m) are effective to snails. Other molluscocides (e.g. based on tin) are tested too. The pond is filled with water through a sieve (mostly made from synthetic web called Uhelon) installed directly on an inlet pipe. The sieve must be regularly controlled and cleaned. This is a very simple way preventing the snails to reach the pond. These mechanical, physical and chemical methods of snail control can be suitably combined with biological method. In such case, 500 – 600 individuals per 1 ha of two-year-old carp (over 500 g) and/or three-year-old tench are stocked into pond. This stock frequently consumes small juvenile snails. In the USSR, black carp is stocked in water bodies to control large quantities of intermediate host snails. Fry of herbivorous fish fed in troughs for several days prior the stocking is stocked into ponds at the turn of June and July. In this period, the stock carp and tench are fed with highly effective feed rich in animal protein to prevent the losses.
Therapy is not yet realized in practice.
Acanthocephalosis
Prevention is based on regular examination of health state of stocked fish and even fish from flowing waters from which the ponds are fed. A main aim of prevention is to prevent the introduction of parasites. Careful drying and/or disinfection of pond bottom with 2.5 t.ha-1 of burnt lime or 0.5 – 0.6 t.ha-1 of chlorinated lime applied immediately after the fishing, controls the eggs and intermediate hosts. Recently an increase in pond fish culture intensity seems to be an important limiting factor of carp neoechynorhynchosis occurrence. It was recognized that an occurrence of intermediate hosts is very sporadic in intensively managed ponds with dense stocks.
Therapy was worked out against neoechinorhynchosis of carp. Carboneum tetrachloratum and paraffin oil in the same ratio is applied in a single dose 1 ml per 1 kg of fish in feed.
Fish Diseases Caused by Parasitic Crustaceans
This group of diseases is represented mainly by the following parasitoses: ergasilosis, lernaeosis and argulosis.
Prevention is based on the excluding infected fish, both from culture and coarse water, to enter the fish culture facilities. Water sources used to supply the fish culture facilities must be under strict control. Thorough drying and/or disinfection of pond bottom with burnt lime or chlorinated lime is a method controlling the eggs and larvae of parasites.
Therapy of ergasilosis and lernaeosis is very difficult. Long-term bath in preparations based on trichlorphone proved to be only temporary effective on nauplia and copepodite stages under higher water temperature (20°C); the same bath is uneffective on adult parasites if applied under lower water temperature. Immersion bath in lysol is recommended for re-fished fish. Long-term bath in preparations based on trichlorphone can be applied directly in pond to cyprinids as argulosis treatment.
Hirudineosis
Prevention - disinfection of pond bottom with burnt lime (2.5 t.ha-1) controls the occurrence of leeches (Piscicola geometra). To prevent the entering of infected coarse fish into the fish culture environment is also an effective method. Another way of prevention consists of creation the unsuitable conditions for leeches in pond which means an eradication of hard littoral vegetation, stones and tree branches serving as a shelter for leeches and/or a substrate for cocoons with eggs.
Therapy is applied primarily in cyprinids. A single application of trichlorphone-based preparation directly into pond is an effective method of leeches control. Blue vitriol (CuSO4.5H2O) in dose 0.05 mg.1-1, i.e. 0.5 kg.ha-1 of pond for water column 1 m on average, applied 3 – 4 times repeatedly in a two- to four-week period is recommended as a control of leeches in carp ponds by some authors. An immersion lime and/or lysol bath, and as the case may be, short-term bath in sodium chloride can be used for leeches control in re-fished fish. It must be taken into mind, that leeches drop off from fish after bath and they are able to recover again in suitable conditions. The salt bath is less effective. From brood fish, the leeches can be removed mechanically by pincette.
Recomended literature
Bauer O.N., Musselius V.A., Strelkov Ju.A. (1981): Diseases of pond fishes. Izd. Legkaja i piščevaja promyšlennost, Moskva, pp. 318 (In Russian)
Lucký Z. (1986): Diseases of important fishes. SPN, Praha, pp. 201 (In Czech).
Prost M. (1989): Fish diseases. Warszawa, PWRiL, pp. 460 (In Polish).
Reichenbach-Klinke H.H. (1980): Krankheiten und Schädigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, pp. 472.
Schäperclaus W et al. (1979): Fischkrankheiten. Akademie-Verlag, Berlin, pp. 1089.
Svobodová Z., Zajíček J. (1989): Control of fish protozoases. In: Lom J., Dyková I. Protozoal parasites of important fishes. SZN, Praha, pp. 102 (In Czech).
ON 46 6809 Antiparasitic and antimycotic bath of fish. ÚNM, Praha, pp. 14 (In Czech).