NMFS, SEATTLE
10 OCTOBER 91
by Dr. Supis
LIST WIDTH (2) SHIMADEU GLC w/3 m packed SS column
ANALYSIS PARAMETER FILE 2


A. INTRODUCTION
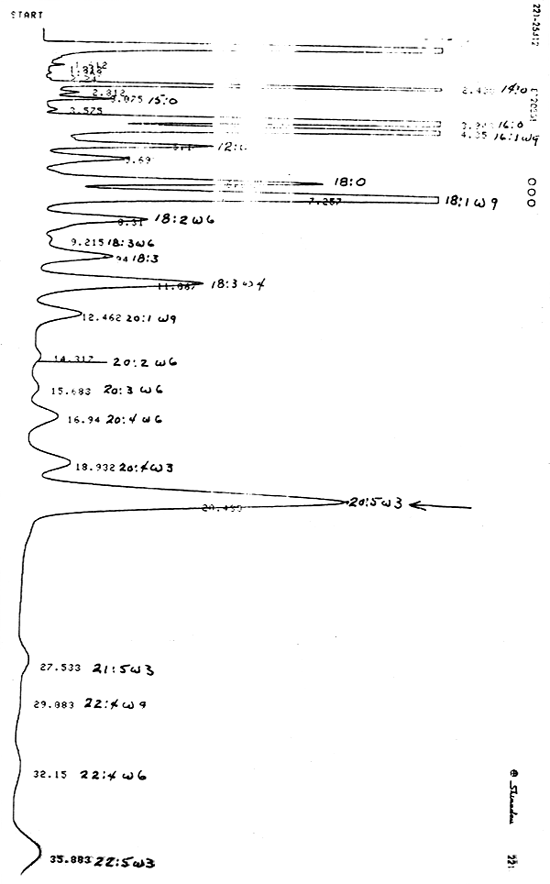
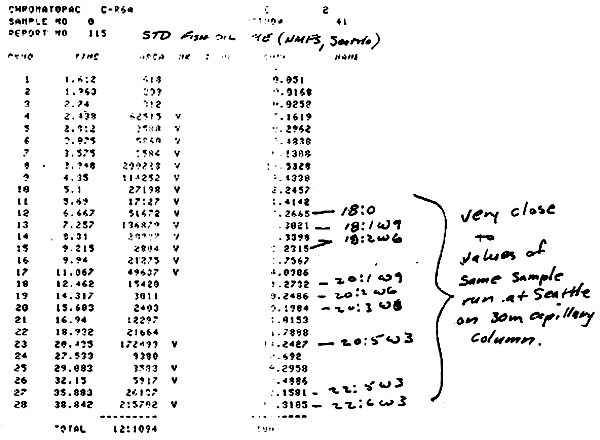
Lipid requirements of fish and shrimp are poorly defined, but lipid needs and functions in growth and physiology are growth and life determinants. General knowledge exists on the need for fats in the diet of warm water fish and shrimp, but quantitative levels for optimum growth and health under various environmental conditions encountered in aquaculture are not known. Since it is known that W-6 and W-3 type fatty acids are needed for proper membrane function, current feed formulations include 1–3% of these fatty acids in the added fat. Fish and shrimp can use neutral lipid sources for energy, thus sparing protein in the diet for growth, but exact levels for various species and growth conditions are only estimated. Protection of labile fatty acids in the diet and in the tissues requires adequate extra cellular and inter cellular antioxidant systems, and these need to be investigated for various fat sources used in aquaculture feeds. Dividends from better knowledge of amount and type of lipid to be used, and for proper protection of these important compounds will promote more economical diets for aquaculture and will assure more positive and reliable aqua-industrial development in Thailand.
B. OBJECTIVES
C. LITERATURE REVIEW
The current state of knowledge on fat and sterol requirements of fish and shrimp will be reviewed with special emphasis on the lipids present in the 10 selected diet oils and on proper protection for essential fatty acids in diet formulations and on fish/shrimp tissue protection. Physiological functions of both neutral lipids and essential fatty acids reported in fish and other animals will be included together with assay techniques to determine both lipid intermediates present in feeds and in growing animals. From the literature survey, obvious voids in knowledge will furnish specific work units for high priority investigations.
D. METHODS
E. NEEDS
F. RELATION WITH OTHER PROJECTS
The lipid research programme will be an integral part of the NIFI nutrition and diet development programme. It will complement projects in vitamin and amino acid research and in feed development technology. In addition it will interface with other NIFI and NICA projects in fish/shrimp health for improved aquaculture in Thailand.
G. PHASING OF WORK
A barchart indicating scheduling of phases of each work unit will be maintained to show progress and achievement of goals and objectives of the overall lipid research programme.
H. REFERENCES FOR LITERATURE
A complete list of citations for the literature review will be submitted with titles and complete authorship for articles listed.
I. BUDGET
A complete budget estimate together with allocation segments requested will be submitted according to budget class accounting listed in accompanying, sheets 5, 6, 7 of the NIFI Project Proforma form.
I. BUDGET
| 01 | PERSONNEL (salaries) | MANMONTHS: | BAHT. |
| a) principal researcher name: | ........ | ............ | |
| b) co-researchers names: | ........ | ............ | |
| c) technicians names: | ........ | ............ | |
| d) labourers numbers/functions: | ........ | ............ | |
| e) others namely: | ........ | ............ | |
| Total personnel | ........ | ............ |
| 02 | BENEFITS (subsidies like houserent, public supplies, insurances, etc.) | |
| a) | ............ | |
| b) | ............ | |
| c) | ............ | |
| Total benefits | ............ | |
| 03 | SUPPLIES & MATERIAL | |
| a) feedstuffs and feed | ............ | |
| b) chemicals | ............ | |
| c) supplies | ............ | |
| d) other (books, etc.) | ............ | |
| Total supplies & material | ............ | |
| 04 | EQUIPMENT | |
| a) | ............ | |
| b) | ............ | |
| c) | ............ | |
| d) | ............ | |
| Total equipment | ............ | |
NATIONAL INLAND FISHERIES INSTITUTE - RESEACH PROJECT PROFORMA
| 05 | RENT & FACILITIES COSTS (other than those on Station) | |
| a) car/truck | ............ | |
| b) pumps, motors | ............ | |
| c) power/water | ............ | |
| d) other | ............ | |
| Total rent & facilities costs | ............ | |
| 06 | TRAVEL | |
| a) national | ............ | |
| b) international | ............ | |
| Total travel | ............ | |
| 07 | CONTRACTURAL SERVICES | |
| a) computer | ............ | |
| b) maintenance of equipment | ............ | |
| c) sub–contracts - analyses | ............ | |
- services | ............ | |
- other, namely: | ............ | |
| Total contracted services | ............ | |
| 08 | PUBLICATION COSTS | |
| a) reports/manuscripts | ............ | |
| b) scientific journals (page charges) | ............ | |
| c) reprints | ............ | |
| Total publication costs | ............ | |
| TOTAL DIRECT COSTS | ............ | |
| 09 | INDIRECT COSTS | |
| a) Station overhead – 20 % of direct costs | ............ | |
| b) NIFI–tax – 5 % of direct costs | ............ | |
| TOTAL PROJECT COSTS | ............ | |
| J. ALLOCATIONS REQUESTED | ||
| a) quarter one | ....................BHT. | |
| b) quarter two | ....................BHT. | |
| c) quarter three | ....................BHT. | |
| d) quarter four | ....................BHT. | |
| K. RECOVERY OF COSTS (INCOME) | ||
| a) sales of fry | ................. | |
| b) sales of fingerlings | ................. | |
| c) sales of foodfish | ................. | |
| d) sales of feed | ................. | |
| e) sales of other products/services, namely: | ................. | |
| Total recovery of costs | ................. | |
(C range 5–50 ppm)
A. Cold Extraction (for C1, C2, C3 MP)
Take 1 gm sample
Add to 10 total with 5% Metaphosphoric Acid
Homogenize in Polytron 1 minute
Centrifuge hispeed 5–10 min (until clear)
Filter thru 0.45 Um disc
Inject 10 mL for assay in HPLC
B. Hot Extraction for (C1, Coated C, C2, C3 MP)
Take 1 gm sample
Add 5% Metaphosphoric acid to 10 gm
Homogenize in Polytron 1 minute
Pour into 100 mL beaker, weigh, (cover with cover glass)
Heat in microwave 30 seconds medium heat
Stand 5 minutes, make back to weight with water
Centrifuge until clear
Filter thru 0.45 Um disc
Inject 10 mL for assay in HPLC
C. TCA Extraction (for C1, C2, C3 MP)
Take 1 gm sample
Add 5% TCA to 10 gm
Homogenize in Polytron 1 minute
Centrifuge until clear
Filter thru 0.45 Um disc
Inject 10 mL for assay in HPLC
Demonstration of use of the Waters HPLC using tandem NOVAPAK 150 mm C18 columns and a mobile phase of 0.1 M sodium acetate plus 200 mg Na2EDTA and 0.17 mL of n–octylamine per liter was completed with Dr. Wimol. Standards of C1 and C2 were prepared and run for time storage stability tests. C1 in water decreased in activity from 90 to less than 30% in one day at ambient temperature. C2 in water solution was stabile at 95–100% activity for at least 5 days. C1 in mobile phase was stabile for one working day (6 hours) but lost over half activity within 24 hours. C1 in 5% metaphosphoric acid solution was stabile for at least 3 days retaining greater than 90% activity. Standards of 10 ppm C1 were used and injections of 10 uL (100 ng C1) were measured on the HPLC yielding 6000 to 7000 area units per ng of C1 injected. Feed mash containing 1000 ppm coated C1 were assayed with 3 extraction techniques to determine best method for feed samples. Raw extruded feed and final dried feed pellets were also extracted and assayed.
Assay results were as follows :
Routine assays of mash feed, raw extruded feed, and dried finished feed could be run on tandem NOVAPAK columns when cold 5% metaphosphoric acid was used to extract and stabilize C1. Coated C1 was fairly stabile in mash mixtures but when water and extrusion plus drying final pellet was done, this C source rapidly degraded, and only 10% of original C1 material was present in the dried pellet. Pellet residue life was not measured as HPLC was replumbed for amino acid analysis until the BONDAPAK tandem columns were delivered.
Calculate sample size (divide by sample wt.)
= ppm C1 in sample
Example
BONDAPAK C18 Column C Assays
Mobile phase : 0.1 M NaAc + 200 mg EDTA + .17 mL n-octylamine per Liter
HPLC conditions : Isocratic flow elution rate 1.5 mL/min
Detector 254 nm. Temperature : ambient
Tandem columns : 4 × 300 mm u BONDAPAK C18
Retention times : C1 @ 4–5 min. C2 @ 8–9 min. C3 @ 20–22 min.
| C1(ug/g) | C2(ug/g) | C3(ug/g) | C3TP(ug/g) | |
| Trout Liver | 15– 25 | 15– 30 | ND | ND |
| Trout carcass | 10– 30 | 50– 150 | ND | ND |
| Trout feed (1) | 30– 100 | 5– 10 | ND | ND |
| Salmon feed (2) | 5– 20 | 80– 100 | ND | ND |
| Salmon feed (3) | 30– 50 | 5– 10 | 60–80 | ND |
| Trout feed (4) | 20– 40 | 5– 10 | 40–60 | 40–60 |
| Salmon Liver | 20– 50 | 20– 30 | ND | ND |
Note: In addition to C1, C2, peaks, trout liver shows another peak midway between C2 and C3MP retention times which, upon treatment with acid, rapidly diminishes with commensurate rise in C1 peak.
(1) Feed with 1000 mg C1 added per kilogram mash before pelleting.
(2) Feed with 100 mg C2 added per kilogram mash before pelleting.
(3) Feed with 100 mg C3 added per kilogram feed.
(4) Feed with 200 mg C3TP added per kilogram mash before pelleting.
A. Water soluble vitamin requirements for warm water fish and shrimp have not been established for quantitative levels to be added to feeds. Deficiency signs have been described for C1 thiamin, pyridoxine, pantothenic acid and niacin which are similar to those reported for salmonds and channel catfish. Vitamin supplements for feeds generally follow NRC recommendations with added amounts for safety. New analytical techniques are now available at NIFI to determine levels in feeds and in fish tissues to give a better assessment of vitamin status in animals on different aquaculture feeds. Therefore it should be possible to determine more exact recommended levels for these vitamins for these expensive feed supplements. Dividends from examination of critical health status vitamin needs in the feed related to health status in the fish and shrimp should enhance aquaculture production in Thailand both in lowered feed costs and in improved fish/shrimp production. Priorities for vitamin investigation should be C, thiamin, pyridoxine pantothenic acid, E, niacin and K. This national program should be an integral part of the Research & Development efforts of NIFI for the next 5 years with ammendments in direction and emphasis made on annual review of progress.
B. OBJECTIVES OF THE VITAMIN RESEARCH PROGRAMME include :
C. LITERATURE REVIEW :
The current literature should be reviewed as to vitamin requirements and functions reported for fish and shrimp species.
D. METHODS
Individual work units will be developed to determine what specific vitamin requirements and feed supplement levels are necessary to guarantee good production and sound health. Growth experiments should be coupled with tissue and clinical chemistry to determine response to different diet treatments. Experimental design will allow statistical analysis of date to determine reliability of results.
E. NEEDS
Specific needs for each work unit will be developed specifying fish/shrimp stock, facilities, (ponds, tanks, lab equipment, etc.), materials (feed, chemicals, supplies) and staff requirements (manhours of labour).
F. RELATION WITH OTHER PROJECTS
The vitamin research efforts are an integral part of the nutrition Research & Development programme and will complement both field and laboratory efforts in lipid and amino acid research to provide sound data on feeds formulation and use in aquaculture in Thailand.
G. PHASING OF WORK
A barchart of approved work units within the vitamin research program will be posted at headquarters and in offices of principal investigatives.
H. REFERENCES
A complete listing of references used in developing the project and work units will be maintained.
I. BUDGET
The budget will be developed according to line items listed in the budget proforma attached.
I. BUDGET
| 01 | PERSONNEL (salaries) | MANMONTHS: | BAHT |
| a) principal researcher name: | ........ | ............ | |
| b) co-researchers names: | ....... | ............ | |
| c) technicians names: | ....... | ............ | |
| d) labourers numbers/functions: | ....... | ............ | |
| e) others namely: | ....... | ............ | |
| Total personnel | ....... | ............ |
| 02 | BENEFITS (subsidies like houserent, public supplies, insurances, etc.) | |
| a) | ............ | |
| b) | ............ | |
| c) | ............ | |
| Total benefits | ............ | |
| 03 | SUPPLIES & MATERIAL | |
| a) feedstuffs and feed | ............ | |
| b) chemicals | ............ | |
| c) supplies | ............ | |
| d) other (books, etc.) | ............ | |
| Total supplies & material | ............ | |
| 04 | EQUIPMENT | |
| a) | ............ | |
| b) | ............ | |
| c) | ............ | |
| d) | ............ | |
| Total equipment | ............ | |
NATIONAL INLAND FISHERIES INSTITUTE - RESEARCH PROJECT PROFORMA
| 05 | RENT & FACILITIES COSTS (other than those on Station) | |
| a) car/truck | ............ | |
| b) pumps, motors | ............ | |
| c) power/water | ............ | |
| d) other | ............ | |
| Total rent & facilities costs | ............ | |
| 06 | TRAVEL | |
| a) national | ............ | |
| b) international | ............ | |
| Total travel | ............ | |
| 07 | CONTRACTURAL SERVICES | |
| a) computer | ............ | |
| b) maintenance of equipment | ............ | |
| c) sub-contracts - analyses | ............ | |
- services | ............ | |
- other, namely: | ............ | |
| Total contracted services | ............ | |
| 08 | PUBLICATION COSTS | |
| a) reports/manuscripts | ............ | |
| b) scientific journals (page charges) | ............ | |
| c) reprints | ............ | |
| Total publication costs | ............ | |
| TOTAL DIRECT COSTS | ............ | |
| 09 | INDIRECT COSTS | |
| a) Station overhead - 20 % of direct costs | ............ | |
| b) NIFI-tax - 5 % of direct costs | ............ | |
| TOTAL PROJECT COSTS | ............ | |
| J. ALLOCATIONS REQUESTED | ||
| a) quarter one | ......................BHT. | |
| b) quarter two | ......................BHT. | |
| c) quarter three | ......................BHT. | |
| d) quarter four | ......................BHT. | |
| K. RECOVERY OF COSTS (INCOME) | ||
| a) sales of fry | ...................... | |
| b) sales of fingerlings | ...................... | |
| c) sales of foodfish | ...................... | |
| d) sales of feed | ...................... | |
| e) sales of other products/services, namely: | ...................... | |
| Total recovery of costs | ...................... | |