The major feed resources for ruminants in developing countries are the fibrous residues from cereal crops and pasture or cut grasses (both usually low in N and low in digestibility) from waste lands.
The insistence on judging these feeds as “low quality roughage” based on their low metabolizable energy content and therefore negligible net energy for production (ARC, 1980) when fed as the sole diet has created the “myth” that these feeds cannot support even low levels of production. This in turn suggests that in diets for productive ruminants, these must be replaced by “high energy-density feeds and protein” such as grains and oilseed meals. These concepts are being challenged because it has been recognized that the forages are only “low quality” because the nutrients arising from fermentative and intestinal digestion are not balanced particularly to meet the specific nutrients needed for the “building blocks” of tissue synthesis, milk production or growth (Preston and Leng, 1985). This means that an energy supplement per se is not the logical supplement to animals on these feeds and a supplement should provide specific nutrients to balance the absorbed products of digestion to meet the animal's requirements.
As the quantities of nutrients required to correct an imbalance appear to be relatively small the term “catalytic supplement” has been coined so as to emphasize the role of specific nutrients in increasing efficiency of use of the basal feed. More importantly, however, the term emhasizes that these supplements should be effective in small quantities.
It must be emphasized that a deficiency of an essential nutrient in any animal results in an inefficient use of the available energy. This is because an animal must rid itself of excess energy through wasteful cycles of metabolism or/and by reducing feed intake. Thus the intake of a basal diet increases when nutrient availability is balanced to meet requirement, by supplementation. The effects of supplementation are thus magnified by an increased efficiency of feed utilized and an increased feed intake. This results in a highly efficient use of a scarce resource. At times, a supplement that contains the current balance of nutrient is used highly efficiently and more efficiently than it would be used by pigs or by poultry (see Preston and Leng, 1985).
Rumen fermentation
Fibrous feeds are necessarily utilized by fermentative digestion in the rumen. This is achieved by a mixed group of microorganisms residue in the rumen. The animal depends on the products of microbial metabolism which include the microbial cells (largely protein) and the short chain acids, acetic, propionic and butyric (usually produced in the appropriate proportions 70, 20, 10).
The key to efficient use of fibrous feeds lies in providing the rumen microorganisms with any deficient nutrients. Usually, on diets based on crop residues the primary limitation is fermentable-N (i.e. N source readily converted to ammonia) followed by a range of nutrients including sulphur, calcium, phosphorus and other macro and micro-nutrients.
Supplying urea to diets based on crop residues increases digestibility, feed intake and the amount of microbial protein produced relative to VFA energy (see Leng 1981). Although supplying urea with crop residues is generally recommended throughout countries such as India, it has not been a generally accepted strategy because of the occasional death of animals from ammonia toxicity resulting from improper supplementation.
The introduction of a molasses/urea block to provide urea and a wide range of nutrients to tethered ruminants fed crop residues, is having a remarkable impact on ruminant production particularly in India (see Leng 1984), and its use is now being extended into many countries, particularly in Africa.
Creating an efficient rumen resolves only some of the problems of feeding animals on diets based on crop residues. The intake of the basal diet and in particular the efficiency of utilization of the feed is still constrained because the products of fermentative digestion do not meet the requirements of the animal for the quantities of, or the balance of amino acids, glucose and long chain fatty acid needed for growth of the foetus or lactation. Supplements to overcome this imbalance should provide protein and starch with a capacity to escape rumen fermentation and to be digested and absorbed from the small intestine, and lipid which is hydrolyzed in the rumen, the long chain fatty acids (LCFA) being absorbed from the intestines.
Requirements for bypass protein
Simply stated, the animal requires more amino acids than are provided by fermentation in the rumen. Supplying a protein meal that escapes rumen fermentation and provides the animal with extra amino acids may have the following effects:
at times it increases total feed intake (see Leng et al. 1974) and
it increases the efficiency of conversion of feed to gain (see Preston and Leng, 1985)
Protein meals that break down in the rumen may also stimulate microbial growth and digestibility of for instance straw-based diet. See Leng this Symposium).
Requirements for long chain fatty acids (LCFA) and glucose
The role of LCFA in the nutrition of ruminants fed crop residues has been neglected.
In summary, LCFA can be deposited in tissue growth at a very high efficiency (Lindsay, 1983). Where little fat is present in a diet the animal must synthesize the LCFA for tissue growth from acetate (or butyrate) which is energy expensive and is therefore an inefficient growth process. In addition to this relatively inefficient use of absorbed substrate there is also need to oxidize glucose to provide the co-factors needed in the synthesis of LCFA in adipose tissue (Vernon 1981). For each gramme of fat deposited in tissue growth about 0.9 g of glucose is oxidized (Preston and Leng, 1985) and as glucose is in limited supply in ruminants (Leng, 1970) because of the degradation of carbohydrates in the rumen, the availability of glucogenic materials may at times be a major constraint that reduces the efficient conversion of feed to gain.
For milk fat synthesis there is also an obligatory need for dietary LCFA which is efficiently used in milk synthesis (Linzell, 1968), but the need for glucose oxidation for the synthesis of milk fatty acids (from C6 - C16) is reduced by the presence of other mechanisms for co-factor (NADPH2) synthesis (see Baumann and Davis 1975). Nevertheless it appears that considerable glucose must be oxidized for fat synthesis. Further, all lactose in milk arises from blood glucose and in toto the need for glucose is greatly increased in the lactating ruminant.
The need for glucose for fat deposition in tissue growth and for fat and lactose synthesis in milk production is influenced markedly by the level of fat in the diet and depending on the quantity of dietary fat glucose availability may be a primary factor influencing the efficiency of utilization of a feed for production (see Preston and Leng, 1984).
The pregnant uterus depends on glucose metabolism for much of its energy needs and it may use as high as 85% of the glucose available (Setchell et al. 1972). That glucose is critical for efficient use of the products of fermentative digestion has been argued by Leng and Preston (1976), Preston and Leng (1980, 1984, 1985); Kronfeld (1976, 1982).
Conclusions on supplementation
Where straws or dry pasture are the main feed available, the productivity of ruminants can be stimulated markedly by feeding practices that ensure an efficient rumen function and also supply and balance the nutrients to meet the particular productive function. The former can be achieved through the use of molasses/urea multi-nutrient block and the latter by providing a mixture of bypass protein, bypass starch and lipid. Some practical suggestions for achieving this are given below.
Bypass proteins: These are best supplied by any heat-treated protein (such as vegetable protein that has been solvent extracted). Forage legumes that have been sun cured may also be a useful supplement but the level of bypass protein is uncertain and possibly quite low but they do provide a source of fermentable-N.
Bypass starch: The grains with a capacity to avoid rumen fermentation include maize, rice and sorghum whereas wheat, barley and oats appear to be almost completely digested in the rumen (Waldo, 1973). Rice polishings containing broken rice may be a good source of bypass starch (Elliott et al. 1978).
Lipid: Although not well researched, adding oil to a fibrous diet is almost always detrimental because the oil is rapidly hydrolyzed in the rumen, and the resultant long chain fatty acids are absorbed onto the fibre and decrease its accessibility to microbial attack and hence reduce digestibility (see Palmquist, 1984). Calcium soaps of long chain fatty acids however are insoluble and appear to be a potential means of supplementing straw based diets (which are inherently low in fat) with long chain fatty acids. This is at present under study in my laboratories.
Examples of “correct” supplementation of straw based diets: The level of production of cattle (300 kg liveweight) given a molasses/urea block, 0.4 kg of a bypass protein, 0.5 kg of rice pollard (19% fat) and ad libitum rice straw was at 365 g/d a highly significant growth rate (see Table 1).
Improving productivity through enhancing digestibility of crop residues
Once rational supplementation of cattle on fibrous feeds has been achieved, undoubtedly the level of production is then “limited” by the low digestibility of the basal feed and the slow exit rate of indigestible residues from the rumen. Further increases in productivity depend on increasing the digestibility of the basal feed. This is feasible because a considerable amount of the straw dry matter is potentially fermentable but is apparently protected from microbial attack by formation of lignocellulose bonds or by physical exclusion of microbes by the lignin covering fermentable substrate.
A variety of physical and chemical treatments is available for improving the potential rate and extent of degradability of fibrous feeds by ruminants. The principal methods use alkalis of which the most widely studied is sodium hydroxide. The method is impractical in almost all countries, but ammoniation using gaseous ammonia or treatment by ensiling with urea, depending on location, may be practical. Both compounds can increase straw digestibility by 5 to 10 units (see Sundstol and Owen, 1984), sufficient to take straw into the class of “high quality feeds” when supplemented according to the principles discussed. This is illustrated by the results of research conducted in both Thailand and Australia (see Tables 1 and 2).
Increasing digestibility of straw by chemical treatment whilst attractive, is often expensive in both doller terms and resource use (urea ammonia gas, plastic sheets) and in the time and effort needed to prepare the stacks. To date very few smallholders are utilizing this approach.
The advent of the widespread use of a molasses/urea block to small farmers allows access to their cattle and the opportunity to manage many aspects of their nutrition and health without the active participation of the farmer. Thus if digestibility of fibrous feeds could be increased by increasing the efficiencies of rumen fermentation by say a chemical additive this may have enormous application to small farmers through the molasses block technology. In the same context manipulation of the rumen microbial contents to increase the ratio of protein to energy, P/E ratio or the glucogenic energy propionate to energy ratio, G/E ratio, will considerably effect the level of supplementation with bypass protein or starch needed to balance nutrient availability.
Increasing digestibility by manipulation of the rumen microbes
The organisms responsible for fibre digestion in the rumen include bacteria, protozoa and fungi. The actual and relative biomasses of each group are a function of the availability of fermentable N, soluble sugar, starches, fibre or protein in the feed. Protozoa occur at all times in the rumen but their population densities increase at the expense of other microbes when increasing levels of soluble sugars or starches are included in a diet.
Recent research of the role of protozoa in the rumen has been quite surprising. In the absence of protozoa, both the fungal (see Table 3) and the “free floating” bacterial pool size (Hungate, 1966) apparently increased. In studies from my laboratories digestibility of fibre increased in the rumen of sheep fed diets of oaten chaff or straw (Table 4). Rumen fungi, which are highly fibrolytic (see Bauchop, 1985) are particularly affected by the absence of protozoa in the rumen. We have observed (Bird and Leng, 1985) that on dry pasture growth and wool growth is increased in unfaunated as compared to faunated sheep (Table 5). There are also indications from European studies (although with only small numbers of sheep (Demeyer et al., 1981) that defaunation of sheep on alkali treated straw based diets increased growth rate by 37% (Figure 1).
Increasing protein to energy ratio in the products of fermentative digesta
It appears to be proven that the removal of protozoa (the major predator on bacteria) from the rumen increases the microbial growth yield in the rumen and this leads to increased productivity where animals have high population densities of protozoa (see Bird and Leng, 1985, Table 5). The effects of faunating (i.e. adding protozoa to the rumen) animals that have never had protozoa in the rumen on protein flow the intestines of sheep is shown in Figure 2.
Increasing glucogenic energy to VFA energy in the products of fermentative digestion
There are now a number of chemicals that can change the microbial ecosystem to produce more propionic acid in the rumen (see Chalupa, 1984), but some unfortunately decrease feed intake. All these chemicals have a major application where glucose availability is the primary constraint to production. However, research is required with these chemicals with ruminants on fibrous feeds, where most other nutritional constraints (i.e. fermentable N and protein) have been removed.
Substitution feeding
Undoubtedly supplementation of straw based diets with grain based concentrates is inefficient because:
starch is fermented more rapidly than fibre and where fermentable N is marginal in a diet, the faster growing starch-digesting microbes will compete effectively for available nutrients (i.e. NH3) with fibrolytic organisms and reduce fibre digestion;
rapid fermentation of starch lowers rumen pH which reduces the growth of fibre digesting organisms (Terry et al, 1969); and
starch is readily stored by protozoa, which increase in numbers when starch is added to a diet, probably lowering the digestibility of fibre but in addition reducing the microbial growth yield.
The substitution of straw by concentrate means that concentrates are used highly inefficiently. Two possibilities exist either singly or in combination to increase the efficiency of use of concentrate.
the use of buffers or compounds which decrease fermentation rate. This could include addition of alkali salts, but perhaps much more economic is the feeding of 2–4% of the concentrate as an expanding clay (Na-bentonite) which decreases fermentation rate of starch and in this way maintains rumen pH high.
The other possibility is through the control of protozoa, which increases the amount of protein available to sheep and cattle (see Bird and Leng, 1985) and possibility also increases digestibility of fibre.
Balancing critical nutrients in a straw based diet and ensuring an efficient rumen system can lead to low to moderate levels of production. If these same feeds are treated to increase digestibility the rumen is manipulated to increase digestibility, intake is increased and with critical supplementation, high levels of production can be achieved at least comparable with that of “good quality” temperate pastures.
The future for ruminant production in developing countries lies in maximizing productivity from the available resources with only minimum importation of feed supplements.
Increasing fibre digestibility by increasing the ‘free floating’ pool of microbes
Rate of colonization of feed, may be the limitation to the rate of digestion. Juul-Nielsen (1981) suggested that a small amount of easily digested fibre mixed with a straw based diet will increase i) the free floating pool of microbes, and ii) rate of colonization of feed, and therefore increase total feed intake. Likely, easily digested fibre sources include beet pulp, citrus pulp and leguminous hays. The effects of such supplements will only be obvious through an increased feed intake due to a more rapid breakdown of the fibre of the basal diet.
Increasing fibre digestibility through genetic manipulation of microbes
This is a field that is being explored; there appears to be little doubt that cellulolysis by bacteria is variable and often dependent on a number of influences such as ammonia concentration in the rumen and the presence of growth factors from the diet. Hungate (1966) discovered the microorganism Clostridium lockhedi which when tested was 15 times more active in cellulose degradation than “normal” rumen organisms.
The selection and/or modification of bacteria and fungi to develop high cellulase or ligno-cellulase activity is being studied in my laboratories. The possibility of transferring a higher capacity to digest fibre between microbes and to normal rumen residents may lead to great increases in productivity of ruminants from straw based diets as indicated by the comparison between productivity of animals on treated or untreated straw (Table 1). The rationale for this approach is that manipulating the rumen may eventually become more easily applied and more “economic” than manipulating the digestibility of the feed by alkali treatment.
The likely success of the development of such genetically modified organisms will depend to a major extent on creating support systems that will ensure their survival and growth in the rumen. The support systems may be designed so as to be provided via a molasses urea block and will therefore have immediate application where this technology is already in place.
As discussed above improving microbial activity will be ineffective unless this is accompanied by rational supplementation but both approaches when combined will have enormous effects on animal productivity in developing countries.
REFERENCES
ARC. 1980 The nutrient requirements of ruminant livestock. Commonwealth Agricultural Bureaux, Farnham Royal, UK.
Bauchop, T. 1985 Rumen anaerobic fungi and the utilization of fibrous seeds. In: Biotechnology and recombinant DNA technology in the Animal Production Industries, Reviews in Rural Science, No.6 (Eds. R.A. Leng, J.S.F. Barker, D.B. Adams and K.J. Hutchinson) University of New England Publishing Unit, Armidale, pp 118–123.
Baumann, D.E. and Davis, C.L. 1975 Regulation of lipid metabolism. In: Digestion and Metabolism in the Ruminant (Eds. I.W. McDonald and A.C.I. Warner) University of New England Publishing Unit, Armidale, pp 496–509.
Bird, S.H. and Leng, R.A. 1984 Further studies on the effects of the presence or absence of protozoa in the rumen on liveweight gain and wool growth of sheep. British Journal of Nutrition (accepted)
Bird, S.H. and Leng, R.A. 1985 Productivity responses to eliminating protozoa from the rumen of sheep. In: Biotechnology and recombinant DNA technology in the animal production industries, reviews in rural science, No. 6 (Eds. R.A. Leng, Barker, J.S.F., Adams, D.B. and Hutchinson, K.J.) University of New England Publishing Unit, Armidale pp 109–117.
Bird, S.H., Hill, M.K. and Leng, R.A. 1979 The effects of defaunation of the rumen on the growth of lambs on low-protein high energy diets. British Journal of Nutrition 42:81–87
Chalupa, W. 1984 Manipulation of rumen fermentation. In recent advances in animal nutrition (Eds. W. Haresign, and D.J.A. Cole) Butterworths, London. pp 143–160.
Demeyer, D.I., van Nevel, C.J. and van de Voord, G. 1981 The effects of defaunation on the growth of lambs fed three urea containing diets. Archives fur Teirernahrung 32:595–604.
Elliott, R., Ferreiro, H.M., Priego, A. and Preston, T.R. 1978 Rice polishings as a supplement in sugar cane diets: the quantities of starch (glucose polymers) entering the proximal duodenum. Tropical Animal Production 3:30–35
Hungate, R.E. 1966 The rumen and its microbes. Academic Press, New York.
Juul-Nielsen, J. 1981 Nutritional principles and productive capacity of the Danish straw mix system for ruminants. In: Maximum Livestock from Minimum Land (Eds. M.G. Jackson, F. Dolberg, M. Hague and M. Saadullah) Bangladesh Agricultural University, Mymensing pp 287–299.
Kronfeld, D.S. 1976 The potential importance of the proportions of glucogenic, lipogenic and aminogenic nutrients in regard to the health and productivity of dairy cows. Advances in Animal Nutrition and Animal Physiology 7:7
Kronfeld, D.S. 1982 Major metabolic determinants of milk volume, mammary efficiency and spontaneous ketosis in dairy cows. Journal Dairy Science 65:2204–2212
Leng, R.A. and Preston, T.R. 1976 Sugar cane for cattle production. Present constraints, perspectives and research priorities. Tropical Animal Production 1:1–22.
Leng, R.A., Nolan, J.V. and Kempton, T.J. 1977 Non-protein nitrogen and bypass proteins in ruminant diets. AMRC Review No. 33, November pp 1–21.
Leng, R.A. 1981 Modification of rumen fermentation. In: Nutritional limits to animal production from pasture (Ed. G.B. Hacker) Proceedings of an International Symposium held at St. Lucia Queensland.
Leng, R.A. 1984 The potential of solidified molasses-based blocks for the correction of multi-nutritional deficiencies in buffaloes and other ruminants fed low quality agro-industrial by-products. In: The use of nuclear techniques to improve domestic buffalo production in Asia. IAEA Vienna pp 135–150.
Leng, R.A., Davis, J. and Hill, M.K. 1984 Estimation of by-pass protein based on wool growth. Animal Production in Australia. 15 pp 431–433
Lindsay, D.B. 1983 Growth and fattening. In: Nutritional Physiology of Farm Animals. (Eds. J.A.F. Rook and P.C. Thomas) Longman, London. pp 261–313.
Linzell, J.L. 1968 The magnitude and mechanisms of the uptake of milk precursors by the mammary gland. Proceedings Nutrition Society 27: 44–52.
Palmquist, D.L. 1984 Use of fats in diets for lactating dairy cows. In: Fats in Animal Nutrition (Ed. J. Wiseman) Butterworths, London pp 357–381.
Preston, T.R. and Leng, R.A. 1980 Utilization of tropical feeds by ruminants. In: Digestive Physiology and Metabolism in Ruminants. (Eds. Y. Ruckebush and P. Thivend) MTP Press, Lancaster pp 621–640.
Preston, T.R. and Leng, R.A. 1984 Supplementation of diets based on fibrous residues and byproducts. In: Straw and Other Fibrous Byproducts as Feed (Eds. F. SundstØl) and E. Owen) Elsevier Press, Amsterdam pp 373–413.
Preston, T.R. and Leng, R.A. 1985 Matching livestock systems to available feed resources. ILCA, Addis Ababa.
Setchell, B.P., Bassett, J.M., Hinks, N.T. and Graham, N.McC. 1972 The importance of glucose in the oxidative metabolism of the pregnant uterus and its contents in conscious sheep with some preliminary observations on the oxidation of fructose and glucose by fetal sheep. Queensland Journal of Experimental Physiology 57:257–266.
Sudana, I.B. and Leng, R.A. 1985 Effects of supplementing a wheat straw diet with urea or urea-molasses block and/or cottonseed meal on intake and liveweight change of lambs. Animal Feed Science Technology (submitted)
SundstØl, F. and Owen, E.C. 1984 Straw and other fibrous by-products as feed. In: Straw and Other By-products as Feed (Eds. F. SundstØl and E.C. Owen) Elsevier, Amsterdam.
Terry, R.A., Tilley, J.M. and Outen, G.E. 1969 Effect of pH on cellulose digestion under in vitro conditions. Journal of Science Food and Agriculture 20:317–320.
Veira, D.M., Ivan, M. and Jui, P.Y. 1984 The effects of ciliate protozoa on the flow of amino acids from the stomach of sheep. Canadian Journal of Animal Science 64 (Suppl.):22–33.
Vernon, R.G. 1981 Lipids in the adipose tissue of ruminant animals. In: Lipids in the Adipose Tissues of Ruminant Animals (Ed. W.W. Christie) Pergamon Press, London pp 280–362.
Waldo, D.R. 1973 Extent and partition of cereal grain starch digestion in ruminants. Journal of Animal Science 37:1062–1074.
Wanapat, M., Duangchan, S., Pongawote, S., Anakewit, T. and Tongpanung, P. 1985 Effects of various levels of concentrate fed with urea-treated rice straw for purebred American Brahman yearlings. In: 5th Annual Workshop of the Australian-Asian Fibrous Agricultural Residues Research Network (Ed. R.W. Dixon) Melbourne University Publishing Unit, Melbourne (in press).
| Straw preparation | Supplement (protein meal) | Growth rate (g/d) |
|---|---|---|
| None | 0 | 38 |
| 0.4 | 365 | |
| 0.8 | 292 | |
| 1.2 | 306 | |
| Treated with | 0 | 236 |
| 3% NH3gas | 0.4 | 497 |
| 0.8 | 601 | |
| 1.2 | 639 |
| Supplementation (kg/d) | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Liveweight gain (kg/d) | 470 | 840 | 930 |
| Voluntary intake | |||
| Treated rice straw | 5.8 | 6.2 | 4.4 |
| Concentrate | 0.9 | 1.7 | 2.6 |
| Total | 6.7 | 8.0 | 7.0 |
| Efficiency of feed utilization | |||
| Feed/gain (kg/kg) | 14 | 10 | 8 |
| Conc./gain (kg/kg) | 2 | 2 | 2.8 |
| Diet | ||||
|---|---|---|---|---|
| Untreated straw | Ammoniated straw | |||
| +P | -P | +P | -P | |
| Sporangia (× 103/cm2 24 h | 2.54a | 5.02b | 5.55a | 9.84b |
| Zoospore (× 104/ml) 2 h after feeding | 4.77a | 16.25b | 7.89a | 17.77b |
Means with different superscripts are significantly different (P<0.05)
| Experiment 1 Untreated straw | Experiment 2 Ammoniated straw | |||
|---|---|---|---|---|
| +P | -P | +P | -P | |
| In vivo | ||||
| Digestibility (%) | ||||
| OM | 43a | 48a | 53a | 57b |
| In sacco | ||||
| DM Disappearance (%) | ||||
| at 24 h | 28a | 31b | ||
| at 48 h | 36a | 40b | ||
| at 24 h | 41a | 47b | ||
| at 48 h | 52a | 61b | ||
| Cotton wool cellulose | ||||
| at 24 h | 51a | 52a | 39a | 48b |
| at 48 h | 81a | 91b | 71a | 77b |
Means with different superscripts are significantly different (P<0.05)
| Year | Number Animals | Study Period (weeks) | Bodyweight gain | Wool growth g/d | ||
|---|---|---|---|---|---|---|
| +P | -P | +P | -P | |||
| Ewes | ||||||
| 1982* | 32 | 23 | -48 | -48 | 3.6a | 4.4b |
| 1983 | 39 | 23 | 67 | 73 | 6.6 | 7.0 |
| 1984 | 37 | 52 | 8 | 0 | 7.5 | 7.5 |
| Weaner Lambs | ||||||
| 1983 | 49 | 16 | 85a | 98b | 7.2 | 7.6 |
* dry season was extended by low rainfall
Values with different superscripts significantly different at P <0.05
| Diet (gN/kg) | Treatment | DM Intake (g/d) | Bodyweight gain (g/d) | Wool growth Patch wt (g) |
|---|---|---|---|---|
| Study No. 1 Six-week study (Leng, 1979) | ||||
| 22 | Faunated | 390 | -11a | 0.93 |
| Defaunated | 450 | 37b | 1.42 | |
| 25 | Faunated | 650 | 75a | 1.53 |
| Defaunated | 690 | 133b | 1.92 | |
| 27 | Faunated | 650 | 146 | 1.82 |
| Defaunated | 690 | 159 | 2.62 | |
| 30 | Faunated | 750 | 179 | 2.54 |
| Defaunated | 740 | 154 | 3.17 | |
| Study No. 2 25-week study (Bird and Leng, 1984) | ||||
| 20 | Faunated | 870 | 122 | 8.0a |
| Defaunated | 890 | 135 | 10.8b | |
| 29 | Faunated | 870 | 122 | 7.9a |
| Defaunated | 930 | 132 | 11.0b | |
Values with different superscripts significantly different at p<0.05
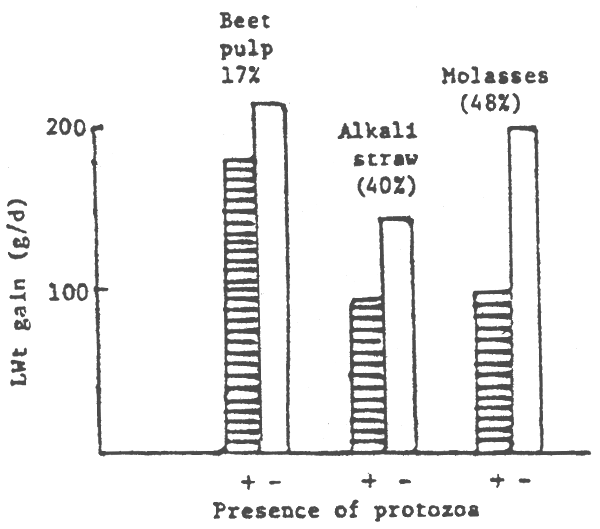
Figure 1. The effects of defaunation of lambs on a variety of diets on liveweight gains (Demeyer et al 1982)
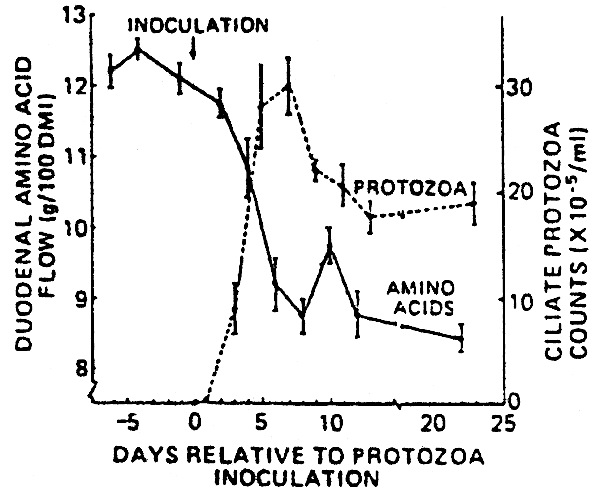
Fig. 2 Changes in duodenal amino acid flow, •—•, and rumen ciliate protozoa counts, •---•, relative to time of inoculation with ciliate protozoa. Values are the mean and SE for six sheep.