1.0 INTRODUCTION
Oyster is a mollusc with a soft un-segmented body protected by two permanent hard shells which increase in size as the animal grows. This marine bivalve belongs to the family Ostereidae which comprises three genera, namely: Ostrea, Crassostrea and Pyncnodonta. There are more than 100 known species of oysters, but only several species are widely cultivated (Quayle, 1980).
Oysters are nutritious food organisms, rich in protein, minerals and vitamins. Their importance as food has helped numerous countries culturing them, to build up foreign exchange earnings. In Korea, for instance, oyster exports in 1982 contributed to about 54% to the total export of canned marine products to Canada, Australia, Holland and Sweden. This is also attributed to the successful culturing of oysters in the Southern sea area of the Korean Peninsula. The Philippines was a significant exporter of oyster to Singapore until the early 1980's. About 180,000 kg of oyster meat valued at Peso 215 million were exported to Singapore in 1980. Foreign market for Philippine oysters include Canada, United States, Netherlands, Switzerland, Jordan, Kuwait, Saudi Arabia, Bahrain and Trust Territory of the Pacific Islands.
Oyster culture is one way of producing food from the sea by farming suitable waters and estuaries where hydrographic conditions favour oyster growth. It lends itself as a mean of providing artisanal occupation to coastal communities either as a principal mean of livelihood or to augment overall income. The latter is true in coastal areas where fishing and aquaculture activities supplement each other; when the sea becomes too rough for fishing, then the small-scale fisherman can turn to his oyster farm to be able to meet his family needs. In addition, oyster farming, if widespread, can help ease fishing pressure in over-fished waters as it diversifies the income sources of fishermen.
Unfortunately, however, several countries in South and Southeast Asia and the Pacific are still engaged in experimental oyster culture and are beset by major constraints such as lack of trained personal/experts (Bangladesh); extreme hydrographic conditions (Burma); non-availability of local species (Fiji); low demand, lack of trained personnel, siltation and red-tides (Malaysia and Indonesia). Thailand is facing the problem of seed supply and low-nutrient waters (Table 1).
TABLE 1. Status of oyster production in South and Southeast Asia and the Pacific.
| Country | Status | Major Constraint |
|---|---|---|
| Bangladesh | Experimental | Lack of trained personnel; exports |
| Burma | Experimental | Extreme hydrographic conditions |
| China | Highly developed | Need mechanization |
| Fiji | Experimental | No local species available for culture |
| Indonesia | Experimental | Low demand; lack of trained personnel; no leasing arrangements; red tide; |
| Malaysia | Experimental | siltation |
| Philippines | Developed | |
| Singapore | None | |
| Sri Lanka | None | |
| Tahiti | None | |
| Thailand | Developed | Limited seed supply; low-nutrient water; |
| Source: | Bivalve Culture in Asia and the Pacific. In: Proceedings of a workshop held in Singapore 16–19 February 1982. Edited by F. Brian Davy and Michael Graham. |
Lack of trained personnel is identified as one major constraint in the expansion of the oyster culture industry. For a prospective oyster culturist, the knowledge of oyster biology as related to culture, various systems of culture and their relative efficiencies, hydrographic conditions favoring high production, importance of pollution-free water, local availability of broodstock/seed and health regulations relating to molluscs, are often the keys to a successful culture operation.
2.0 BIOLOGY OF OYSTERS
Knowledge of the biological characteristics of the cultured oyster species, is an essential and basic requirement for anyone intending to channel efforts and capital into this aquaculture practice.
2.1 Anatomical features
The oyster is a bivalve whose lower left shell is usually cupped and upper right shell generally flat. The two shells are hinged by an elastic ligament at the umbonal or anterior end. The hinge force tends to spring open the two valves, which is opposed by the action of a simple adductor muscle, attached internally on each shell. The shell is usually nacreous inside and horny outside. In addition, the oyster shell is usually fluted when grown on a hard surface while smooth when grown in muddy bottoms. The salinity level also affects the structure of the shell. At high salinity values the shell is usually quite hard while the opposite is true at low salinities (Fig. 1 A).
Internally, the body of the oyster is ventrally and dorsally covered by the mantle which secretes the shell. The mouth is located towards the umbonal end. Surrounding the mouth are labial palps consisting of four leaf-like appendages responsible for the selection and rejection of food particles. Along the ventral part of the body are the gills composed of four long finely ridged, beige-colored appendages. On the gill surface are hair-like structures called “cilia” which create an incoming current. The gills and cilia are responsible for collecting food and oxygenation of the blood. The digestive system consists of a mouth, oesophagus, stomach, crystalline style, liver and anus which are located above the adductor muscle. The liver or digestive diverticula is a series of branched tubes which turn light brown, black or dark green in colour depending on the animal feeding actively. Posterior to the adductor muscle is the heart which is very simple, consisting of one auricle and one ventricle. The nervous system is even simpler, being made up of three nerve cells. The reproductive organs or gonads are the ovaries in the females and testes in the males, which become greatly enlarged when fully matured (Fig. 1 B).
2.2 Breeding habits, larval development and setting
Knowledge of the breeding habits, larval development and setting behavior of the oyster is important in terms of the collection of oyster seed.
Oysters belonging to the genera Ostrea and Crassostrea are quite distinct from each other with regard to their breeding habits. Ostrea species exhibit alternation of sexuality within one spawning season. The eggs, after they have been released from the gonad, are retained in the mantle cavity, while the sperms are extruded externally. The eggs are fertilized by the sperms from outside and half of the larval life takes place in the shell before they are released to the open water.
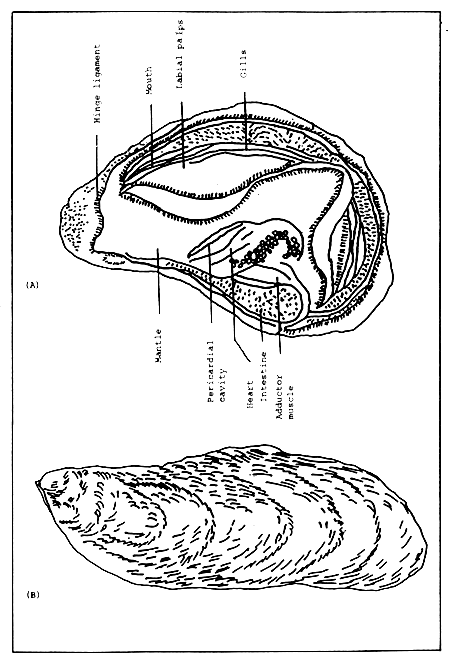
Figure 1. (A) External appearance of an oyster shell and (B) internal anatomy of an oyster.
On the other hand, oysters of the genus Crassostrea change sex after one spawning season. The sperms and eggs are released into the seawater, either all at one time or in small amounts over a long period of time. The eggs are fertilized externally and all subsequent developmental stages occur in the open water.
During the planktonic larval stages, the oysters are at the mercy of the environment (Fig. 2). Swimming is aided by the ciliated velum. As metamorphosis progresses, the oyster larva crawls by means of an extensible foot and explores for a suitable substratum. As soon as it is able to locate a suitable substratum, it attaches itself by means of the byssus gland. Once attached, the oyster becomes a spat. Oyster larvae usually prefer clean and hard surfaces, and this is the kind of cultch the oyster culturist should provide.
The oyster culturist should be aware that sexually mature oysters can be stimulated to spawn by manipulating water parameters such as salinity and temperature. Rapid changes in salinity or temperature can “tickle” sexually mature oysters to release their gametes.
2.3 Food and feeding habits
Oyster food consists of phytoplankton (diatoms and dinoflagellates), copepod larvae, protozoans and detritus (Fig. 3). In estuaries, where the hydrographic conditions are favorable, plankton is abundant and therefore the oysters tend to perform well. During the dry season the seawater salinity and temperature tend to increase and the oysters are found to be thin and watery, suggesting a low supply of food.
Oysters are filter-feeders, and are considered obligatory herbivores. Adult oysters are fixed to a hard substrate and therefore the food availability depends entirely on the natural food present in the surrounding waters. Thus, oysters completely depend on tidal currents for obtaining food; low current velocities and limited flushing hamper growth and fattening.
2.4 Predators, parasites and fouling organisms
Several organisms prey on the larvae and adult oysters.
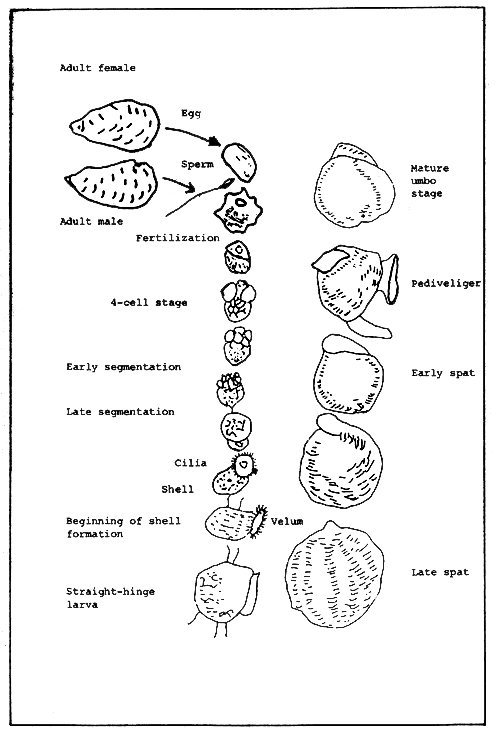
Figure 2. Developmental stages of an oyster.
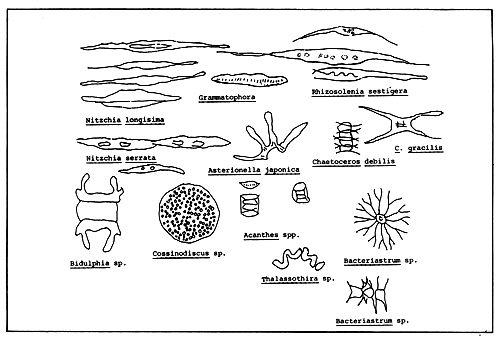
Figure 3. Organisms typically consumed by oysters and other bivalves.
The planktonic oyster larvae face the danger of predation from plankton-feeding animals, including adult oysters. Adult oyster predators include fish (sting ray, bat ray, porcupine fish, toad fish, seabream and black drum); crabs (mud crab and rock crab); snails (conch and drill); starfish, and flatworms (Fig. 4).
Some organisms may cause irritation problems, while other may compete for food. Boring sponges, boring seaworms, boring molluscs, pea crabs and fouling organisms are typical oyster pests.
Parasitism by flatworms and tapeworms in oysters has been recorded in several parts of the world. However, they seldom cause mortality, but may interfere with growth and reproduction.
Diseases in oysters are caused by viruses, fungus and protozoans. In Australia, large specimens of Crassostrea commercialis have been affected by the so-called “opening disease” or “winter mortality” during prolonged spells of high salinity, significantly affecting the industry considering that the oysters reach marketable size in 3–4 years. The causative organisms closely resembles the Haplosporidian, Minchinia nelsoni which affects oysters in the United States. A mesenchymal tumor has also been found to disturb the Pacific oysters.
Fouling organisms can become a major problem when the oysters are permanently submerged as in the floating culture method. Typical foulers are barnacles, mussels, tunicates, polychaetes and hydroids. Heavy fouling may cause severe oyster mortality. There are several ways of minimizing the disastrous effect of fouling. One is to identify the annual fouling sequence and then culture oyster around the period, or to culture the oysters away from fouling areas, or destroy the fouling organisms.
3.0 CULTURE
3.1 Species cultured
Out of 100 known oyster species, only several are widely farmed. The most widely cultured species are Crassostrea angulata and Ostrea edulis in Europe. Other species are C. iredalei (Philippines); C. gigas (Japan, Korea, West coast of the United States and Canada); C. commercialis (Australia); C. brasiliana (East coast of Southern South America); C. chilensis (West coast of South America); C. margaritacea (South Africa); C. gasar (along the central West coast of Africa). C. gigas has been recently introduced into France, England, Morocco, Australia and New Zealand (Table 2).
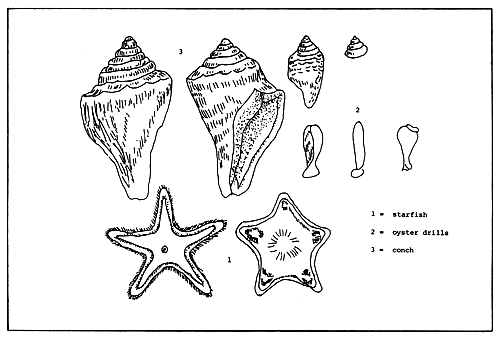
Figure 4. Natural oyster predators.
TABLE 2. THE PRINCIPAL CULTURED SPECIES OF OYSTERS, THEIR DISTRIBUTION AND CHARACTERISTICS
| SPECIES | COUNTRIES WHERE CULTURED | SPAWNING SEASON | SPAWNING TEMPERATURE (°C) | INCUBATION PERIOD | DURATION OF LARVAL PERIOD AND TIME OF SETTING | DEPTH AND TIDAL ZONE INHABITED |
| Crassostrea angulata (Portuguese oyster) | Portugal, Spain, Atlantic coast of France; experimentally in Japan, Tuirisia, and California | Summer | 20 or more | - | 15–20 days | Intertidal; in estuaries where current is strong |
| C. commercialis (Sydney rock oyster) | Australia, from southern Queensland to eastern Victoria and New Zealand | Summer and fall | Peak at 21– 23 | 6 hours | 14–21 days; February-April | From intertidal to 3 m below low tide |
| C. cradelie (slipper oyster) | Philippines | Spring and summer, peaks during rainy season (July-August) | 30–33 | - | 7 days | Intertidal |
| C. gigas (Pacific oyster) | Japan, Korea, Taiwan, Pacific coast of United States and Canada; experimentally in Australia, France, Netherlands, Portugal, Thailand, and United Kingdom | Peaks in Japan: May-June in Inland Sea, August-September in North Japan | Begins at 19– 20, peaks at 23–25 | 5–6 hours | 10–14 days; peak in August | Intertidal |
| C. shizophorae (mangrove oyster) | Experimentally in Cuba and Venezuela | Continuous, peaks May. September in Venezuela | - | - | Continuous set. ting, peaks in July-August in Venezuela, February-April in Cuba | 0.5–3.0 m (intertidal) |
| C. virginica (American oyster) | Atlantic and Gulf coasts of United States, maritime provinces of Canada: experimentally in Japan and California | Long Island Sound: mid. July—early October; Chesapeake Bay: mid. June-mid. October; South Carolina: May-October; Gulf of Mexico: April-November | Begins at 20 | - | 10–21 days | Intertidal to more than 30 m; spawning and spat settling most successful in estuaries |
| Ostrea edules (flat oyster) | Atlantic coast of France, Spain, Netherlands, Great Britain, Japan, United States (Maine and Pacific coast) | June-September in Mor. bihan area of France | 20 or more | 8 days | 12–14 days | Little or no intertidal exposure; in estuaries where current is weak |
Table 2.
| SPECIES | TEMPERATURE TOLERANCE (°C) | SALINITY TOLERANCE (%) | SUBSTRATE | SIZE MARKETED AND GROWING TIME TO THAT SIZE |
| Crassostrea angulata (Portuguese oyster) | About 15–25 | Optimum 20–30; fails to reproduce above 34 | Various; quite tolerant of unbidity | 65 g (including shell); 3 years in France |
| C. commercialis (Sydney rock oyster) | Varies widely | Varies widely | Hard bottom, usually in the shade | 85–100 mm (for consumption on halfshell). 2½ years in North, 3½ years in the South; 50–65 mm long (for shucking), 2–3 years |
| C. eradelie (slipper oyster) | 25–33, possibly wider | Wide, up to 45, spawns at 15 | Usually over mud on plants; cultured over sand; very tolerant of silt | 75 mm diameter, 6–9 months |
| C. gigas (Pacific oyster) | 15–30, optimum for larval development 23–25; best spat sets at 25 or more | Optimum for larval development 23–28 (varies with temperature) best spat sets at 15–18 | Any hard substrate | 30–60 g (including shell), 6– 12 months (Inland Sea), 18 months (North Japan) halfshell size, 2 years |
| C. rhizophorae (mangrove oyster) | 18.4–34.0 | 22–10 (extremes of short duration); optimum 26– 37 | Associated with mangrove roots; very tolerant of turbidity | 75–100 mm diameter, 5–6 months |
| C. virginica (American oyster) | Larvae develop well at 17.5–32.2 | Wide, to at least 32; best larval development above 16.5, some survive at 7.5, maximum for larvae 22.5 | Hard | 75 mm diameter, 4–5 years. Northern Atlantic Coast; 2–3 years, mid. to South Atlantic Coast and Gulf of Mexico |
| Ostrea edulis (flat oyster) | Wide—at least 4–22; optimum 15–20; 100% mortality at 26 sensitive to variation | Usually found above 25 | Hard, not tolerant of turbidity | 65 g (including shell), 75 mm diameter, 4 years |
3.2 Site selection
Wave/wind action. Waters in bays and coves enjoy considerable degree of protection. Information regarding wave and wind pattern of occurrence and intensity is usually useful to determine whether a site is suitable or not.
Salinity. Oysters thrive best in brackish- and full strength seawater. Optimum range is about 17–26 ppt (Blanco et al., 1951). Areas which are prone to flooding or surface run-offs should be avoided.
Natural food supply. There should be an abundant supply of phytoplankton. Plankton sampling and analysis may serve as a good guide in determining the productivity of the area.
Availability of broodstock/seeds. The best area, as far as this criterion is concerned, is one which has a natural population of the species to be cultured. While oysters can be transplanted from one area to another, procurement of broodstock or seedling is difficult and costly, especially if the distance from the source to the farming area is great.
Pollution. It is important that the culture area is free from any form of pollution. Areas which are endangered by chemical, industrial or domestic effluents should be avoided. Oysters are filter-feeders and have the capacity to absorb and accumulate heavy metals (such as zinc, copper and mercury) and pathogenic organisms. In sitting an oyster farm, this aspect of pollution should be seriously considered inasmuch as the lives of both oyster and their consumers, including man, are involved.
Water depth. Water depth should be sufficient for the selected culture method.
Post-harvest and marketing facilities. Oysters are highly perishable, and since local demand is largely for fresh or raw oysters, marketing facilities such as roads, transportation, ice plant and cold storage should be present.
Availability of culture materials.
3.3 Culture methods
Gathering oysters which naturally occur in the wild is not culturing. Oyster culture consists of gathering their seeds and growing them to marketable size. Although techniques and methods of culture can vary from one place to another, the general underlying principles are common to all. Spawning, larval development and provision of food for juveniles and adults are left to nature.
Collectors or cultches are installed on-bottom or off-bottom in order to catch the settling larvae (Fig. 5). Later, the seeds are transferred to the growing or fattening areas. Sometimes the seeds may not be transferred, in which case the seeding area also becomes the growing and fattening area.
Culture methods are generally categorized into on-bottom and off-bottom. Each method has its advantages and disadvantages, thus it is up to the oyster culturist to choose the method most suitable for the selected site and his financial possibility.
Bottom culture. This is growing oysters directly on
the bottom sub-tidally or inter-tidally (Fig. 6).
This method requires a stable, non-shifting bottom
within the correct tidal range (Quayle, 1980). While
bottom culture is the simplest and cheapest, danger of
mortality and stock loss due to predation, siltation
and wave action are greatest. Harvesting is
difficult. In the tropics where potential oyster
areas are largely estuaries of mud and soft bottom,
bottom culture is generally uncertain.
The method simply consists of planting the seedling
directly on the bottom where they are left to grow to
the marketable size.
Disadvantages are: the method is limited to shallow
waters with firm bottom, reduced production per unit
area, high mortality due to siltation and predation
and difficulty in harvesting.
Off-bottom culture. Where bottom conditions are not
suited due to softness, wave action and tidal level,
oysters are held in suspension or off-bottom in
several ways. This method can be costly, however this
is compensated by the rapid growth and high quality of
the cultured oysters.
Off-bottom culture is divided into three methods:
raft, rack and stake.
Raft culture. Oysters are suspended from floating structures such as raft. Oysters maybe held in tray or stringed. The rafts can be of any shape or material and styrofoam, oil drums or polyethylene floats are used the float the raft.
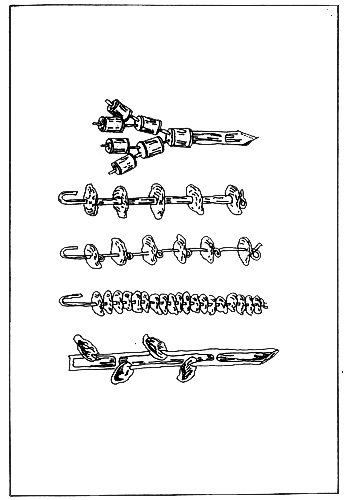
Table 5. Several kinds of oyster spat collectors.
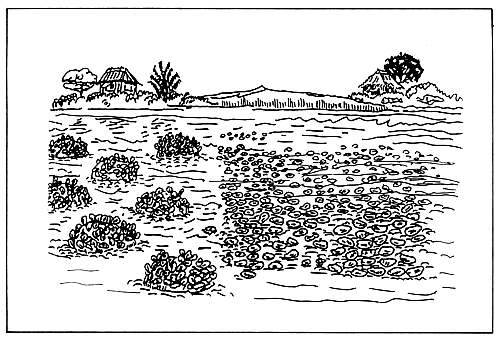
Figure 6. Oyster bottom culture method.
Raft-tray. To grow single, well-shaped oysters for a particular market such as the half-shell trade, the tray method is used. A tray may be made of wire or plastic mesh with a wooden frame, or entirely made of bamboo. Single oysters are laid on trays and allowed to grow until marketable size. This culture method is costly because of the high investment and high cost of maintenance.
Raft-string. Individual pieces of cultch are
strung on galvanized wire, locally woven rope,
synthetic cord or monofilament nylon.
For spat collection, cultches are strung close to
each other, whereas for growing or fattening,
cultches with spats are strung 8–12 inches apart.
The length of the rens depends upon the depth of
water in the culture area and lifting machinery
available.
Rack culture. Racks of wood, bamboo or metal,
imbedded in the ground either sub-tidally or inter-tidally,
are used to hold vertically or horizontally
oysters which are on trays or strings or sticks (Fig.
7, 8 and 9).
This method allows control of biofouling because it
permits oysters to be suspended at a level where they
may become briefly exposed during low tide. Rack
culture is a low-cost technology. Its disadvantage,
however, is that it can be economical only if applied
to a maximum depth of 2–3 meters, as the cost of
operation increases with increasing depth.
The rack-string is very productive, as experienced in
the Philippines. Aside from yielding high volumes per
unit area, the other advantages are: no mortality
from silt, reduced mortality from bottom crawling
predators, rapid growth, ease of harvesting, and
method suitable for shallow waters.
Its only disadvantage is that it is costly and
requires a considerable supply of materials.
Stake culture. Suitable for shallow lagoons which are
too shallow or too soft-bottomed for other culture
methods.
The stakes hold oysters vertically off-bottom. The
stake itself can also act as the cultch, or some other
forms of cultches, such as shells, may be impaled in
or nailed to it. Harvesting is laborious and
difficult.
4.0 HARVESTING AND STORAGE
Harvesting of oysters must be timed when oyster condition is at its best; that is when the meat is full and creamy. Seasonal changes in oyster condition should be determined as a guide for harvesting time.
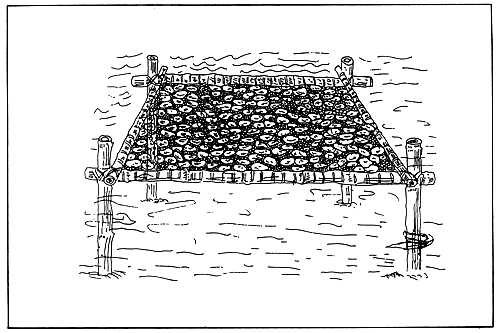
Figure 7. Oyster tray culture method (chicken wire or bamboo tray).
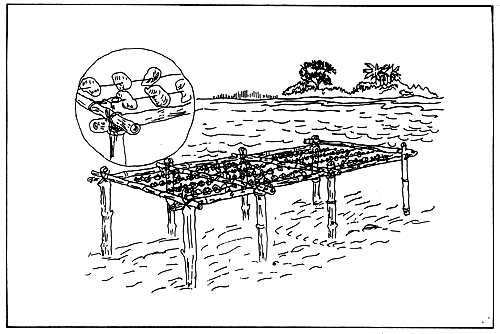
Figure 8. Oyster long-line culture method on a wooden rack.
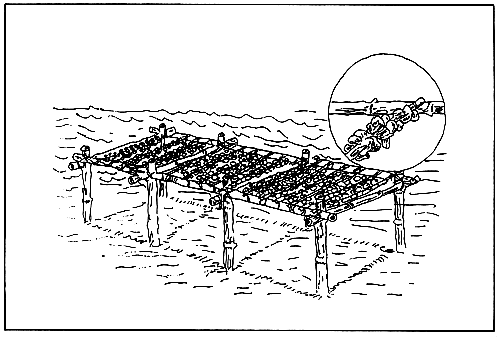
Figure 9. Oyster horizontal rack culture method.
Bottom cultured oysters can be hand-picked or dredged. Oysters in racks or rafts are manually harvested unless the strings or trays are too heavy and require some form of mechanization.
Oysters are best marketed immediately or briefly after harvest, however they may be stored either wet or dry, shell-on or shucked.
Shucked oysters require cooling and safe storage temperature to arrest bacterial and enzymatic decomposition. Shelf life is influenced by storage temperature as shown below:
| Temperature | Shelf life | ||
|---|---|---|---|
| 12 | °C | 3–5 | days |
| 8 | °C | 7–8 | days |
| 1 | °C | 16 | days |
Live oysters can also be stored dry or wet. Wet storage requires un-polluted seawater. When dry-stored, a refrigerated room can extend storage for days or even weeks.
5.0 EQUIPMENT AND MATERIALS
The oysters culturist requires to invest capital to purchase the following equipment and materials (the first three are essential):
Boat for transport.
Culture materials such as rope, wire, nails, bamboo, anchors, floats, buckets, baskets, oyster shucking knife and rubber gloves.
Carpenter's tools.
Optical equipment such as microscope, etc.
Glassware such as watch glasses, graduated cylinder, jars, pipettes, glass slides and cover slips.
Oceanographic equipment: hydrometer, thermometer, salinity refractometer and current drogue.
Land transportation.
Weighing equipment: weighing balance and suspension-type scale.
6.0 ECONOMICS OF OYSTER FARMING
The cost and benefit derived from oyster culture varies from method to method and the type of materials used. An example derived from the Philippine experience, on such variation, is presented in the following table:
Receipts, expenses and measures of profitability for various methods of oyster culture.
| Item | Stake | Hanging | Lattice | Broadcast |
|---|---|---|---|---|
| Average farm size (m2) | 1603 | 5968 | 472 | 3678 |
| Capital investment | ||||
Per farm | 400 | 800 | 203 | 111 |
Per hectare | 2495 | 1340 | 4301 | 302 |
| Total annual receipt (P/ha) | 8942 | 18934 | 19001 | 1556 |
| Total annual expense (P/ha)* | 5711 | 4975 | 9559 | 1408 |
| Net Annual Earnings (P/ha) | 3231 | 13919 | 9442 | 148 |
| Earnings on sales (%) | 36 | 73 | 50 | 10 |
*= Family labor included
Source: Librero et al. (1976)
See also Table 3 and Table 4.
7.0 SANITATION MEASURES AND OYSTER DEPURATION
Oysters, wild or cultured, being filter-feeders can absorb and accumulate chemicals, bacteria and biological toxins from the surrounding waters. Two major types of pollution, that can affect oysters and consumers, derive from industrial activities and sewage discharges.
Industrial effluents can be directly toxic to oysters, or can hamper their physiological activities. Effluent particles can clog the gills and reduce the dissolved oxygen.
Direct discharge from main sewers or drainage from individual or improperly installed septic tanks can be a severe form of pollution.
In case of contaminated growing areas, the following sanitation control measures are necessary:
Table 3. Estimated costs and returns of a one-half hectare oyster farm on the hanging method for one yeara
| Item | Cost ( ) ) | Life (Yr) | |||||
| 1. | Costs | ||||||
| A. | Fixed Cost | ||||||
| 1. | Materials and labor for plot construction of 125 plotsb | 49,250 | 2 | ||||
| a) | 42 pc bamboo poles (“puno”) at  4.50/pole 4.50/pole | (189) | |||||
| b) | 21 pc bamboo poles (“baral”) at  2.25/pole 2.25/pole | (47) | |||||
| c) | 10 pc bamboo poles (“bila”) at  10.00/pole 10.00/pole | (100) | |||||
| d) | 1 kg nail No. 4 at  8.00/kg 8.00/kg | ( 8) | |||||
| e) | Contract labor to prepare plot | ( 50) | |||||
Cost for one plot | 394 | ||||||
| 2. | 1 native banca (dugout) | 1,000 | 3 | ||||
| 3. | Shed | 500 | 3 | ||||
| 4. | Tools and diving paraphernalia | 500 | 5 | ||||
Total fixed cost | 51,250 | ||||||
| B. | Production Cost | ||||||
| 1. | Operating cost | ||||||
| a) | 1 caretaker at  500/mo for 12 monthsc 500/mo for 12 monthsc | 6,000 | |||||
| b) | 440 kaings of empty oyster shells at  4.50/kaingd 4.50/kaingd | 1,980 | |||||
| c) | 240 rolls of nylon rope No. 4 at  12.00/rolle 12.00/rolle | 2,880 | |||||
| d) | contract labor to make “bitin” or collectorsf | 2,190 | |||||
| e) | harvesting charges at  3.00/kaingg 3.00/kaingg | 9,210 | |||||
Sub-total | 22,260 | ||||||
| 2. | Depreciation | ||||||
| a) | plot construction | 24,620 | |||||
| b) | dugout | 330 | |||||
| c) | shed | 170 | |||||
| d) | tools | 100 | |||||
Sub-total | 25,220 | ||||||
| 3. | Miscellaneous | ||||||
| a) | municipal permit | 30 | |||||
| b) | repairs and other expenses | 500 | |||||
Sub-total | 530 | ||||||
Total production cost | 48.010 | ||||||
| II. | Returns | ||||||
| A. | Gross Income: sale of 3,070 kaings at  38.00/kaing 38.00/kaing | 116,660 | |||||
| B. | Net Income: (gross income minus total production cost)h | 68,650 | |||||
| C. | Returns per Peso Invested | 2.43 | |||||
Table 3.
Table 4. Estimated costs and returns of a one-half hectare oyster farm on the stake method for one yeara
| Item | Cost ( )) )) | Life (Yr) | ||||
| I. | Costs | |||||
| A. | Fixed Costs | |||||
| 1. | 1 native banca (dugout) | 1,000 | 3 | |||
| 2. | shed | 500 | 3 | |||
| 3. | tools and diving paraphernalia | 300 | 5 | |||
| 4. | stake and stake preparation | 23,950 | 2.5 | |||
a) 5,000 pc bamboo poles (puno) at  4.50/poleb 4.50/poleb | (22,250) | |||||
| b) hired labor for staking | ( 1,250) | |||||
| c) raft rental and towing charges | ( 200) | |||||
Total fixed costs | 25,750 | |||||
| B. | Production Costs | |||||
| 1. | Operating costs | |||||
a) 1 caretaker at  500/month for 12 monthsc 500/month for 12 monthsc | 6,000 | |||||
b) harvesting charges for 625 kaings at  5.00/kaingd 5.00/kaingd | 3,125 | |||||
Sub-total | 9,125 | |||||
| 2. | Depreciation | |||||
| a) dugout | 330 | |||||
| b) shed | 170 | |||||
| c) tools | 60 | |||||
| d) stake | 9,580 | |||||
Sub-total | 10,140 | |||||
| 3. | Miscellaneous | |||||
| a) Municipal permit | 30 | |||||
| b) Repairs and other expenses | 170 | |||||
Sub-total | 200 | |||||
Total production costs | 19,465 | |||||
| II. | Returns | |||||
| A. | Gross Income: sale of 625 kaings at  38.00 each 38.00 each | 23,750 | ||||
| B. | Net Income: (gross income minus total production cost)e | 4,285 | ||||
| C. | Returns per Peso Invested | 1,22 | ||||
a BFAR, 1981; and oyster farmers of Bacoor, Cavite. Cost data are based on 1981prices.
b Bamboo trunks are staked 1 m apart.
c Caretaking is often provided partially or entirely by the family.
Table 4.
Bacterial examination of the growing waters. The indicator organism used is the human faecal coliform bacteria. An allowable bacterial level fit for human health must be established. The coliform count should be constantly monitored for the information of the consumer.
Sanitary surveys. To identify suitable farming areas.
Strict regulations governing the storage operations of processing plants.
Bacterial examination of the final product at the market level.
Depuration of oysters. Shellfish depuration is based on the knowledge that filter-feeding molluscs remove particles from the surrounding, digest some and discharge some in the form of pseudofaeces (Quayle, 1980). The simplest and cheapest depuration for oysters is to transfer them from a contaminated to an uncontaminated area for 48 hours. Another form is to place the oysters in a holding tank through which pure or purified water is made to flows. The most expensive method is to purify oysters with chlorine, ozone or ultraviolet light (Fig. 10).
Shellfish marketing and consumption can be affected by dinoflagellate blooms or the so-called “red-tides” which causes the paralytic shellfish poisoning symptoms. The safest measure is to refrain from eating oysters during the period of red tide. Toxicity retention can vary.
The economic impact of red-tides can be seen in the loss of employment for those who are directly or indirectly engaged in oyster production, as well as loss of market, international trade, and reluctance to invest in this aquaculture sector.
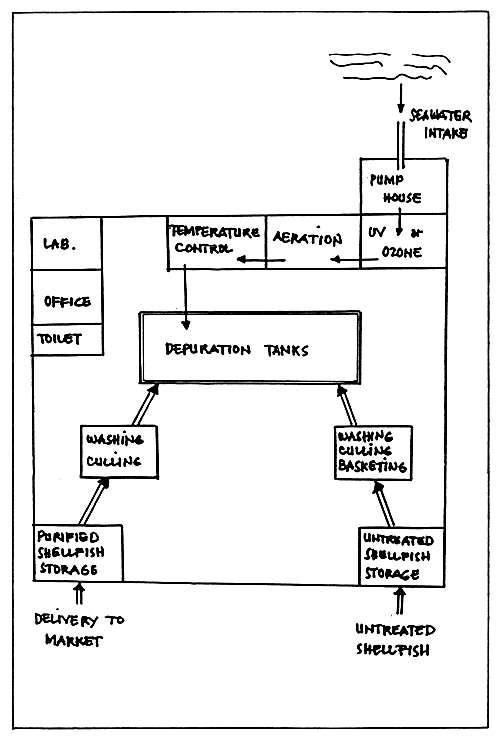
Figure 10. Schematic diagram of a shellfish purification (depuration) plant.
REFERENCES
Ablan, Guillermo, L. 1949. The commercial production of oysters in the Philippines. Bureau of Printing, Manila.
Blanco, G.J. and D.K. Villaluz. 1951. The Cultivation and Biology of Oysters in Bacoor Bay, Luzon. Phil, Journal of Fisheries. Vol. 1.
Choi, I.H. 1983. The Korean Canning Industry. INFOFISH Marketing Digest, No. 5.
Davy, B. and M. Graham. 1982. Bivalve Culture in Asia and Pacific. In: Proceeding of a workshop held in Singapore 16–19 February 1982.
Glude, J., Steinber, M. and R. Stevens. 1982. The feasibility of oyster and mussel farming by municipal fisherman in the Philippines. FAO/UNDP, Manila.
Haven, D.S. and F. Perkins. 1978. Bacterial depuration by the American Oyster (Crassostrea virginica) under controlled conditions. Institute of Marine Science, Virginia.
Korringa, P. 1976. Farming of Cupped Oyster of the genus Crassostrea. Elsevier, Amsterdam.
Quayle, D.B. 1980. Tropical Oysters: Culture and Methods. Ottawa, Ontario, IDRC.
Villaluz, D.K. 1939. Vertical distribution of oysters spats in Bacoor Bay. Phil. Jour. Sci. 70.
Walne, P.R. 1974. Culture of bivalve molluscs. Fishing News Books Ltd. Farnham, Surrey, England. 189 p.
Wilbur, K.M. and C.M. Yonge. 1965. Physiology of Mollusks. Academic Press, New York.
Young, A. and E. Serna. 1982. Bivalve Culture in Asia and the Pacific. Proceedings of a Workshop held in Singapore 16–19 February 1982. Brian F. Davy and Michael Graham (eds.).