J. Olah and F. Pekar
Fisheries Research Institute,
H-5540 Szarvas, Hungary
ABSTRACT
Condensed results of 18 Ph. D studies carried out at the Fisheries Research Institute, Szarvas, Hungary on fish-cum-pig and fish-cum-duck ponds are synthesized to construct the nitrogen cycles of these organic loaded ecosystems. Averages and ranges of 13 water and sediment nitrogen compartments, including dissolved and particulate, combined and free dissolved amino acids as well as dissolved and absorbed sediment ammonia ions, were quantified. All the known nitrogen transfer rates were measured in synchronous study or estimate with nitrogen balance: nitrogen fixation, ammonification, ammonia regeneration, amino acid uptake, ammonia uptake, nitrification, nitrate uptake, nitrate respiration, dentrification. Based on management parameters, compartment and transfer rate measurement, the nitrogen balance and budget were also composed for the experimental ponds and for a fish-cum-duck commercial farm. For the farm nitrogen budget, nitrogen inputoutput data from 20 years were collected and analysed. This analysis emphasizes the importance of the nutrient processing and accumulating nature of the fish-cum-livestock ecosystems in industrialized agricultural landscapes.
INTRODUCTION
The energy cost of one ton fish produced in a recycling system was 367 GJ, equivalent to 8.5 tons of crude oil. Most of this energy was burned to substitute the natural cycling of nitrogen in order to maintain a healthy environment for proper fish growth. In contrast the energy to produce one ton of fish in fish-cum-cattle untrainable ponds in India is only 10 GJ (Olah and Sinha, 1986). Such energy intensive strategies are used in the industrialization of agriculture in most of the developed countries. As a result the production of one ton of wheat consumes 50 times more energy than 50 years ago when during the traditional closed agriculture practice the nitrogen was recycled (Olah et al., 1991). In the so called modern agriculture we burn increasing amounts of oil to let the nitrogen run through crop fields directly to rivers and groundwaters. Subsidies help the agriculture to destroy natural environment. Nitrogen rain above, nitrate poisoned groundwater below, dead rivers alongside and perhaps an alternative agriculture with recycling nitrogen on the horizon.
Fortunately fisherman everytime had an intimate and very tight connection with nature. Even in Western developed countries a carp culturist in France or a salmon restocking culturist in USA would rather operate a capture type fisheries. Only the new trout and catfish industries are more energy-intensive. The old Eastern fish-cum-livestock culture practice perfectly fulfills the principle of the newly emerging alternative agriculture. These fishponds receive and process nitrogen from the environment. The practice explores exclusively the natural resources with production on a very intensive level per unit area.
Unfortunately the quantitative aspect of their nitrogen balance, budget, cycle and metabolism was very poorly investigated. The majority of quantification on nitrogen fixation, ammonification, ammonia regeneration, ammonia uptake, amino acid uptake, nitrification, nitrate uptake, nitrate respiration, denitrification is restricted to natural waters and generated from environmental research projects. The only nitrogen laboratory which was operated and is still existing for simultaneous measurement of almost all nitrogen pathways in fish-cum-livestock ponds was established in our institute, in Hungary. During the last 15 years, 18 Ph.D. thesis were completed on different aspects of nitrogen and fish pond ecosystems. Here, we briefly synthesize our results on the nitrogen metabolism in fish-cum-duck and fish-cum-pig ecosystems. Hundreds of measurements on a particular nitrogen pathway and compartment were condensed to a single figure in order to quantify the unit transfer rate (kg N ha-1 yr-1) from one compartment to another. Details are available in the thesis of my students (Table I) or in papers published mostly in our journal “Aquaculture Hungarica” between 1978 and 1990.
WATER AND SEDIMENT NITROGEN COMPARTMENT
In nitrogen budgeting or cycling models, compartments symbolize the existing molecular form of nitrogen with infinite resolving possibility for their different pools present in water, in sediment, inside a particular species or functional groups of organisms. However, in practice, only few compartments are quantified both in water and terrestrial ecosystems. In fish-cum-duck and fish-cum-pig ecosystems we have selected and monitored 13 compartments in the water column and sediment. A special manual for nitrogen compartment and transfer rate measurements was published with detailed descriptions of glassware, reagent preparation, procedure and calculation (Olah and Janurik, 1985). This standardized methodology offers a real comparative analysis in quantification.
The ranges varied widely between the fish-cumduck and fish-cum-pig ecosystems (Table II). The highest maxima were accumulated either during the peak summer, like Particulate Amino Acid (PAA-N) in the body protein of planktonic organisms, or at the end of the growing season with decreasing sunshine, like NH4-N. The NO3-N maxima were observed at the very beginning of the culture period when ponds were filled with river water. The dominant nitrogen compartments occurred in the sediment of both the fish-cum-duck and fish-cum-pig ecosystems. Almost 2 mg PAA-N g-1 dry sediment were present in the second half of the growing season. This compartment represents the nitrogen content of living and dead organic particles. The average number of bacteria in the sediment of these heavily loaded ecosystems was 8.1×109g-1 dry weight as measured with direct count of epifluorescent microscopy. Calculating with an average bacterial nitrogen content of 2.8 fg N cell-1 we estimate the total nitrogen content of the sediment bacterial biomass to be as small as 22.6 μg g-1 dry sediment. This is around only one percent of the total PAA-N. The nitrogen content of the benthic invertebrates is even less, only 2 μg g-1 dry sediment. Based upon these calculations we concluded that 98.9 percent of the total PAA-N in the sediment is enclosed in dead particles. This compartment serves as an important nitrogen reserve decomposing and releasing dissolved free amino acids (DFAA-N) and ammonia nitrogen. The bulk of this regenerated ammonia is absorbed and accumulated on fine clay particles or on organic complexes forming the second most important nitrogen compartment in these fish-cumlivestock ecosystems.
NITROGEN TRANSFER RATES
In fish-cum-livestock ecosystems the nitrogen cycle operates primarily on nitrogen load, released directly by animals living in close contact with the ponds or introduced indirectly as collected manure if animals live far from the ponds. The organic nitrogen of dissolved and particulate proteins and peptides are cut into small molecules of free dissolved amino acids by extracellular enzymes of bacteria and taken up by them or to a smaller extent by certain physiological groups of algae mostly blue-green algae. Part of the assimilated amino acids is respired and released as NH4-N.C14 labeled amino acids were used to determine the individual amino acid uptake rates while labeled algal protein hydralisate was used to estimate the total amino acid uptake rate (Table III). On average, glycine and glutamic acid uptake rates were richest among the individual acids. The methionine uptake rate was high in the fish-cum-pig ecosystem but low in fish-cum-duck ponds. A significant percentage of the assimilated glycine and glutamic acid up to 64 %, was respired as compared to 13 % of methionine and valine in fish-cum-duck ponds.
The ammonia as the end product of amino acid respiration or as commonly known as the ammonofication together with nitrate as its oxidized form, are the key inorganic nitrogen molecules for the autochtonous protein production utilizing the energy and reducing power of the photosynthetically generated carbohydrates in the ponds. Their uptake rates were measured with Michaelis-menten uptake kinetics. Ammonia was taken up preferably in both the fish-cum-duck and fish-cum-pig ecosystems (Table IV).
Table I. Ph.D theses on nitrogen and fish pond ecosystem supervised by J. Olah and available for reading at the Fisheries Research Institute, Szarvas, Hungary.
| Ph.D Students | Thesis subject |
| Toth. O.E. 1976 | Amino acid and protein cycling |
| Kintzly A. 1978 | Nitrogen fertilization in polyculture |
| ElSamara M.I. 1980 | Nitrogen fixation NH4 and NO3 uptake |
| Abdelmoneim M.A. 1982 | Denitrification |
| Csengeri I. 1982 | Lipids in fish feeding |
| ElSarraf W.M. 1983 | Amino acid uptake and ammonification |
| Eross I. 1983 | Fish feeding and body composition |
| Papp Zs. 1983 | Pond amino acid quantification |
| Janurik E. 1984 | Nitrogen quantification methodology |
| Pekar F. 1984 | Nitrification |
| Farkas J. 1985 | Bacterial populations |
| Talaat K.M. 1986 | Fish population growth |
| Kovacs Gy. 1987 | Domestic sewage fishponds |
| Ayyappan S. 1988 | Waterhyacinth processing fishpond |
| Szabo P. in prep. | Sediment N compartment, diffusion |
| Nezami B.S. in prep. | River nutrient load |
| Esteky A.A, in prep. | Sediment nutrient accumulation |
| Liptak M. in prep. | Bacterial production |
Table II. Water and sediment nitrogen compartments in fish-cum-duck and fish-cum-pig ecosystems - (Ranges and averages).
| Compartments | Fish-cum-duck | Fish-cum-pig |
| Water | ||
| PAA-N (μg N dm-3) | 115–478 | 243–2813 |
| DCAA-N (μg N dm-3) | 47–881 | 105–974 |
| DFAA-N (μg N dm-3) | 0.6–11.3 | 2.3–8.9 |
| NH4-H (μg N dm-3) | 69–893 | 97–4590 |
| NO2-N (μg N dm-3) | 42–132 | 13–278 |
| NO3-N (μg N dm-3) | 227–1530 | 94–1310 |
| Sediment | ||
| PAA-N* (μg g-1) | 1976 | 1750 |
| DCAA-N (μg N dm-3) | 761 | 681 |
| DFAA-N (μg N dm-3) | 23 | 103 |
| NH4-H (μg N dm-3) | 346–3484 | 1099–7499 |
| Absorbed NH4-N* (μg g-1) | 58–181 | 11–122 |
| NO2-N (μg N dm-3) | -70 | 22–107 |
| NO3-N (μg N dm-3) | 30–117 | 64–166 |
Particulate amino acid - PAA; dissolved combined amino acid - DCAA; dissolved free amino acid - DFAA. (Toth et al., 1984 except sediment inorganic N: Szabo and Olah, in prep).
* - of per g dry sediment.
Although the nitrate uptake rate was lower, especially in fish-cum-duck ponds, it was a very important nitrogen pathway transporting daily a significant amount of inorganic nitrogen building material for protein synthesis.
Table III. Amino acid uptake in fish-cum-livestock ecosystems μg dm-3h-1 (ElSarraf, 1983).
| Amino acid | Gross Vmax | Respired % |
| Fish-cum-duck | ||
| Glycine | 6.0 | 45 |
| Methionine | 2.3 | 13 |
| Lysine | 2.4 | 17 |
| Valine | 1.5 | 13 |
| Phenylalanine | 2.0 | 36 |
| Glutamic acid | 6.7 | 64 |
| Protein hydrolysate-N | 9.3 | 22 |
| Fish-cum-pig | ||
| Glycine | 8.5 | 43 |
| Methionine | 10.8 | 21 |
Table IV. NH4-N and NO3-N uptake in fish-cum-livestock ecosystems, μg N dm-3h-1 (ElSarraf et al., 1982).
| Ecosystem | NH4-N Vmax | NO3-N Vmax |
| Fish-cum-duck | 21 | 14 |
| Fish-cum-pig | 24 | 19 |
Table V. Nitrogen fixation, nitrification and denitrification in the water column and sediment of fish - cum - livestock ecosystems kg ha-1 100 day-1
| Nitrogen transfer | Water column | Sediment | Total |
| Fish-cum-duck | |||
| Nitrogen fixation | 7.8 | 65 | 72.8 |
| Denitrification | 36 | 23 | 59 |
| Nitrification | 320 | 462 | 782 |
| Fish-cum-pig | |||
| Nitrogen fixation | 5.7 | 6.1 | 11.8 |
| Denitrification | 30 | 42 | 72 |
| Nitrification | 642 | 361 | 1003 |
(Abdelmoneim et al., 1986; Olah et al., 1987; Pekar et al., 1989)
Biological nitrogen fixation which is an important nitrogen source in many natural waters has only secondary significance in these organic loaded ecosystems (Table V). Filamentous blue-green algae which are the dominant and more successful nitrogenfixing organisms in the water column cannot develop significant biomass here under the grazing and bioturvating pressure of dense fish populations. Moreover, the water column is never deficient of inorganic nitrogen which favours blue-green blooms. The rates of nitrogen-fixation measured with the acetylene reduction method were somewhat higher in the sediment especially in fish-cum-duck ponds. Why is here this level of nitrogen-fixation in these sediments which is full of dissolved ammonium ions as we have quantified with the Reeburgh interstitial extracting procedure (Table II)? Combined inorganic nitrogen and especially ammonium is known to have a negative influence on nitrogen fixation. In the anaerobic fixer, Clostridium pasteurianum, which was the dominant nitrogen fixing organism in these pond sediments, reaching a population density of 106 - 107 cell g-1 dry weight, ammonium represses the synthesis of nitrogenase but dose not affect the activity of the enzyme. In aerobic nitrogen fixers, ammonia both represses the synthesis of nitrogenase and inhibits its activity. To answer the question we speculate that nitrogen-fixation might be a way to remove reduction equivalents from reduced sediments. It is one way to live without oxygen in order to utilize space and to transform nitrogen and energy in such an organic loaded system full of surplus electrons. The electron reception function seems more pronounced ecologically than the commonly considered nitrogen-fixation aspect of this type of bacteria thriving in this waste processing ecosystem.
Bacteria carry out denitrification in oxygen deficient sediments. Denitrifiers perform an electron reception function reducing the nitrate into N2O or N2 gases which leave the system into the atmosphere, decreasing at the same time the electron pool of the sediment. The denitrification rate was measured in intact core sediment with acetylene inhibition procedure. Although the measured rate of denitrification was significant in both ecosystems at least among the outputs of the budget (Table V) it was lower than usually measured in other aquatic systems. Nitrification was the most significant nitrogen transfer pathway in both ecosystems (Table V). In fish-cum-pig ponds the amount of nitrogen recycled in this route surpassed one ton per hectare per year.
NITROGEN CYCLING AND RETENTION
Management parameters, synchronized compartment and transfer rate measurements made the construction of a nitrogen cycle and budget possible (Figs. 1 and 2). In these ecosystems, the driving force is the manure introduced by duck and pig populations and the sun. Besides the manure nitrogen, the river water supply with high nitrate content and the dry and wet atmospheric deposition are significant nitrogen input sources. The contribution of biological nitrogen fixation was significant only in the fish-cum-duck ponds. We do not know yet why this is so. One possible explanation is the higher specific phosphorus content of the duck manure. The cycling of the introduced nitrogen has similar quantitative patterns in both ecosystems. However, the pig fed pond cycle operates on a more intensive level due to the higher nitrogen input introduced by the manure. Pig ponds are higher loaded than duck ponds. The influence of this higher load appears on transfer rates operating both in the sediment and in the water column. The measured rates of amino acid respiration, that is the bacterial ammonification of 329 kg ha-1y-1 in fish-cum-pig and 226 kg ha-1y-1 in fish-cum-duck systems which were considered earlier as the dominant pathway of the ammonium production, forms only the smaller part of the total ammonia regeneration; 774 ± 193 kg ha-1 in fish-cum-pig and 630 ± 163 kg ha-1 in fish-cum-duck ponds were regenerated by invertebrate and fish ammonia releases. This is an important quantitative aspect of the cycle quantified here. Planktonic and benthic invertebrates grazing on algae as well as fish feeding on invertebrates release around 75% of the assimilated nitrogen. There are quantitative findings for natural waters supporting this conclusion (Liao and Lean, 1978; Gardner et al., 1983; Mitamura and Saijo, 1986; Tatrai, 1987). Outstanding pathway of nitrification was quantified as transfer route oxidizing ammonia to nitrate up to one ton ha-1y-1 while producing an equivalent amount of bacterial organic carbon, i.e. 125 kg C ha-1y-1 into the food-chain. During this process 3.4 tons of oxygen was combined and stored to cover the shortages of electron acceptor demand in these waste processing and fish-cum-livestock producing ecosystems. Based on balance calculation almost half of this combined oxygen is respired in the most reduced sediment layers in the process of the recently discovered nitrate respiration pathway. To eliminate as much electrons as possible, special bacterial populations reduce nitrate not only to nitrogen gas but further to ammonia displacing four more electrons per nitrogen atom. This bacterial activity was described from very reduced ecosystems: ricefield sediment (MacRae et al., 1968), anaerobic soil (Buresh and Patrick, 1978), anaerobic coastal marine sediment (Koike and Hattori, 1978: Sorenson, 1978), digested sludge (Kaspar et al., 1981) and bovine rumen (Kaspar and Tiedje, 1981).
Figure 1. Nitrogen cycle in fish-cum-duck ecosystems, kg ha-1y-1. Input and output, cycling.
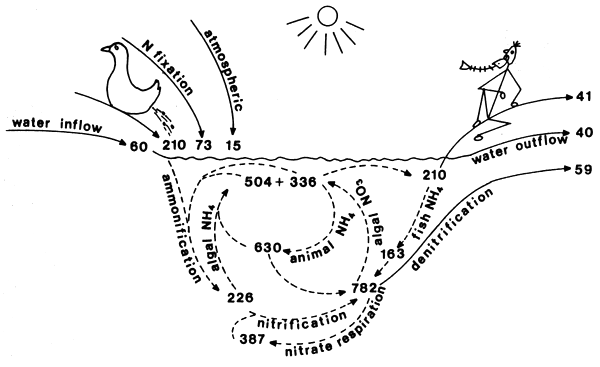
Figure 2. Nitrogen cycle in fish-cum-pig ecosystems, kg ha-1y-1. Input and output, cycling.
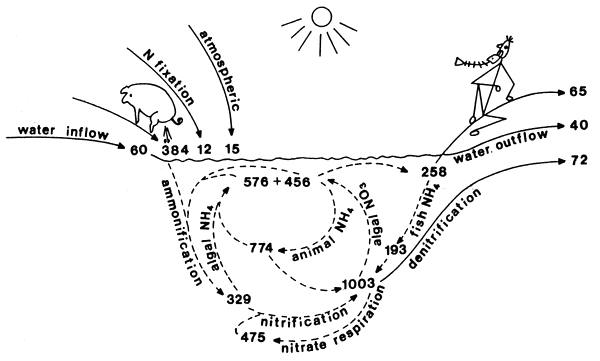
Table VI. Nitrogen budget of 20 years (1970-1990) on our commercial fish-cum-duck-alfalfa-rice farm Horvathpuszta with around 300 ha, tons 20 yr-1.
| Input | Output | ||
| Inorganic fertilization | 212 | Alfalfa yield | 46 |
| Duck feed | 156 | Fish yield | 86 |
| Fish feed | 17 | Rice yield | 64 |
| Manure | 14 | Duck yield | 84 |
| Atmospheric deposition | 90 | Draining water | 170 |
| Filling water | 255 | Denitrification | 236 |
| Alfalfa nitrogen fixation | 210 | Total output, farm-1 | 686 |
| Fish pond nitrogen fixation | 292 | Total output, ha-1y-1 | 0.114 |
| Total input, farm-1 | 1246 | Total input-output, farm-1 | 560 |
| Total input, ha-1y-1 | 0.207 | Total input-output, ha-1y-1 | 0.093 |
Average nitrogen contents used in analysis. Manure: 0.5, fish feed: 1.6, duck feed: 1.9, alfalfa: 2.7, fish: 2.5, rice: 2.4, duck: 2.7 percent.
Sediment harbouring important nitrogen compartments and operating significant transfer routes functions as a nitrogen accumulator. Subtracting the total nitrogen output removed with harvested fish, water outflow and denitrification from the total nitrogen input we receive accumulation values of 218 kg N ha-1 for fish-cum-duck and 294 kg N ha-1 for fish-cum-pig systems. These nitrogen amounts were trapped in the sediment during the growing season. We have not measured or estimated how much nitrogen was denitrified, volatilized or leached after the growing season when sediments become dried and exposed to sun and wind. Definitely there must be significant nitrogen loss during the season of 4– 5 months. To answer this question we have analysed the twenty year nitrogen budget in our commercial fish-cum-duck-alfalfa-rice farm of around 300 ha. The twenty year average value for annual accumulation was only 93 kg ha-1 (Table VI) as compared to the above values accumulated in experimental ponds during the growing season. The leaching, denitrification and volatilization as quantitatively important during the dry period emphasizing the technological importance of this management parameters. The nitrogen budgeting model of this commercial farm was verified with sediment nitrogen measurement. Fortunately the total nitrogen content in the sediments of the commercial farm was studied twenty years ago. The measurements gave a farm average of 1.62 mg N g-1 dry weight (Fabry, 1975). We have reexamined the sediment nitrogen content of the same 19 ponds. The farm average after 20 years increased to 5.10 mg Ng-1 dry sediment. The annual rate of nitrogen accumulation calculated from these measured values was 174 μg Ng-1 dry sediment. This rate of accumulation corresponds well with the budgeted accumulation values calculated for the farm. This analysis emphasizes the nutrient processing and accumulating nature of fish-cumlivestock ecosystems. This function provides important perspectives for nutrient recycling in aquaculture, and also of such practice as an alternative agriculture being important in the industrialized agricultural landscapes in developed countries.
REFERENCES
Abdelmonim M.A.; J. Olah and P.Szabo (1986). Denitrification in water bodies and sediments of Hungarian shallow waters. Aquacultura Hungarica (Szarvas) 5: 133–146.
Buresh R.J. and Patrick W.H.Jr. (1978). Nitrate reduction to ammonium in anaerobic soil. Soil Sci. Soc. Amer. J. 42: 913–918.
ElSarraf. W.M., J. Olah, M.J. ElSamra, M.A., Abdelmoneim and F. Pekar (1982). Inorganic and organic nitrogen uptake in the water and sediment of Hungarian shallow waters. Workshop on Eutroptication of shallow lakes. Modeling, monitoring and management 29 Aug. – 3 Sept. 1982, Veszperm, Hungary.
ElSarraf W.H. (1983). Amino acids and microbial activity in Hungarian shallow waters. -Ph.D. thesis, Budapest, Hungarian Academy of Sciences.
Fabry Gy. (1975). Sediment chemistry of Szarvas fish ponds with aquacultural relation. - MEM Press, Budapest, 92 pp.
Gardener W.S., T.F. Nalepa, D.R. Slavens and G.A. Laird (1983). Patterns and rates of nitrogen release by benthic Chironomidae and Oligochaeta. - Can. J. Fish Aquat. Sci. 40: 259–266.
Kaspar H.F. and Tiedje J.M. (1981). Dissimilatory reduction of nitrate in the bovine rumen: nitrous oxide production and effect of acetylene. Appl. Environm. Microbiol. 41: 705–709.
Kaspar H.F., Tiedje J.M. and Firestone R.E. (1981). Denitrification and dissimilatory nitrate reduction to ammonium in digested sludge. Can. J. Microbiol. 27: 878–885.
Koike I. and Hattori A. (1978). Denitrification and ammonia formation in anaerobic coastal sediments. Appl. Environ. Microbiol. 35: 278–282.
Liao C.F.H. and D.R.S. Lean (1978). Nitrogen transformation within a trophogenic zone of lakes, J. Fish. Res. Board Can. 35: 1102–1108.
MacRae D.C., Ancajas R.R. and Salandanan S. (1968). The fate of nitrate nitrogen in some tropical soils following submergence, Soil Sci. 105: 327–334.
Mitamura O. and Y. Saija (1986). Urea metabolism and its significance in the nitrogen cycle in the euphoric layer of Lake Biwa. IV. Regeneration of urea and ammonia. Arch. Hydrobiol. 107: 425–440.
Olah J. and Janurik E. (1985). Methods to measure nitrogen cycling in shallow waters. - MEM Press, Budapest, 226 pp.
Olah J., Toth L. and M.I. ElSamra (1987). Biological nitrogen fixation in shallow waters. Academic Press, Budapest, 191 pp.
Pekar F., J. Olah and L. Toth (1989). Nitrification in fishponds and in natural waters. II. Nitrification in open waters and in sediment. Vizugyi Kozlemenyek 71: 295–310.
Sorensen J. (1978). Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. - Appl. Environ. Microbiol. 35: 301–305.
Tatrai I. (1987). The role of fish and benthos in the nitrogen budget of Lake Balaton, Hungary. - Arch. Hydrobiol. 110: 291–302.
Toth E.O., J. Olah and Zs. Gy. Papp (1984). Amino acid compartment in the water and sediment of Hungarian shallow water. Aquacultura Hungarica (Szarvas) 4: 111–134.