| FAO Fisheries Synopsis No.92 | FRm/S92 SAST - Shrimp |
prepared by
A.C. SIMPSON, B.R. HOWELL and P.J. WARREN
Ministry of Agriculture, Fisheries and Food
Fisheries Laboratory
Burnham-on-Crouch, Essex, England
Pandalus montagui Leach, 1814
Leach, W.E., 1814, Brewster's Edinburgh Encyclopaedia, 7, (Col - Div, Crustaceology): 432, 436.
Astacus maculatus (Montagu MSS) Leach, 1814 (invalid becaused published in synonymy). Pandalus annulicornis Leach, 1815
1 For a discussion on the nomenclature of this species see Hemming (1965).
Suprageneric
Phylum Arthropoda
Class Crustacea
Subclass Malacostraca
Series Eumalacostraca
Superorder Eucarida
Order Decapoda
Suborder Natantia
Section Caridea
Family Pandalidae
Generic
Pandalus Leach, 1814, Brewster's Edinburgh Encyclopaedia, 7:432. Gender masculine. Type species by monotypy: Pandalus montagui Leach, 1814.
The generic concept as used by Holthuis (1955) has been adopted by the authors. The diagnostic characters employed in his ‘Key to the Pandalidae’ are listed below.
Carpus of 2nd pereiopods consisting of more than 3 joints. No longitudinal carinae on the carapace except the postrostral crest. Rostrum not movable. Eyes well developed, cornea much wider than the eyestalk. Third maxilliped without exopod. Laminar expansion of the inner border of the ischium of the 1st pair of pereiopods wanting or inconspicuous. The first 4 pereiopods with epipods. Arthrobranchs present at the bases of the first 4 pereiopods.
Posterior lobe of scaphognathite acutely produced. Upper margin of rostrum with movable spines only.
Specific
Identity of type specimen
The specimen examined and described by Leach was collected from Zetland (Shetlands), and is now available at the British Museum (Nat.Hist.London) (Dry 267d, Leach cabinet no. 29). This specimen was selected the lectotype of the species.
Type locality: “Zetland” (=Shetland Islands).
Diagnosis
Third maxilliped without exopod. Rostrum with 10 to 12 teeth above and 5 or 6 below, dorsal teeth not extending beyond middle of rostrum; apical spine of antennal scale extending beyond lamellar portion; 3rd abdominal somite smooth dorsally. (Kemp, 1910).
Subjective synonymy
Pandalus levigatus Stimpson, 1853
Pandalus leptorhynchus Kinahan, 1858 (not P. leptorhynchus Stimpson, 1860, not P. leptorhynchus Sars, 1882).
The larval species Boreocaris moebiusi Ortmann (1893) has been incorrectly referred to the synonymy of this species (see Pike and Williamson, 1964).
Artificial key
There is no one key to all the known species of Pandalus: but keys are given to the pandalids occuring in the northeast Pacific and the northeast Atlantic. No key is available to the pandalids of the northwest Atlantic.
Key to northeast Atlantic pandalids (after Kemp, 1910).
Third maxilliped without exopod
Carpus of 2nd pereiopod on right side with many (at least 20) annulations; antennal scale not much narrowed in front, outer edge straight.
Rostrum with 12 to 16 teeth above and 7 below, the dorsal teeth extending well into the anterior third; lamellar portion of antennal scale extending beyond apical spine; a blunt dorsal carina terminating in a tubercle on the 3rd abdominal somite, P. borealis.
Rostrum with 10 to 12 teeth above and 5 or 6 below, the dorsal teeth not extending beyond middle of rostrum; apical spine of antennal scale extending beyond lamellar portion; 3rd abdominal somite smooth dorsally, P. montagui (Fig. 1).
Carpus of 2nd pereiopod on right side with 4 annulations; antennal scale very narrow in front, outer edge concave, P. propinquus.
Third maxilliped with exopod; carpus of 2nd pereiopod on right side with 4 annulations, Dichelopandalus bonnieri.
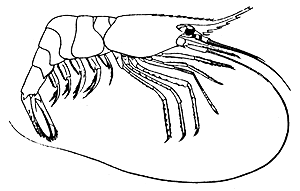
Fig. 1 Pandalus montagui Leach (after Holthuis, 1950).
Key to northeast Pacific Pandalids (after Butler, 1964).
Antennules twice the length of carapace; merus of 3rd maxilliped, and ischium of 1st pereiopod with longitudinally developed laminate expansions, with long bristles, Pandalopsis dispar.
Antennules not longer than carapace; no laminate expansions on merus of 3rd maxilliped or ischium of 1st periopod. (2)
Third segment of abdomen in part compressed and carinated, the carina forming a more or less well-defined lobe or spine in front of the posterior margin. (3)
Third segment of abdomen not compressed or carinated. (5)
Third and 4th segments of abdomen each armed with a median spine of the posterior margin, Pandalus borealis.
Third and 4th segments of abdomen without median spines. (4)
Rostrum unarmed on distal half of dorsal margin, P. goniurus.
Rostrum with spines on distal half, P. jordani.
Dorsal spines not present behind middle of carapace. (6)
Dorsal spines present behind middle of carapace. (7)
Sixth abdominal segment more than twice as long as wide. P. montagui tridens.
Sixth abdominal segment less than twice as long as wide, P. platyceros.
Dorsal spines more than 15 (17 to 21), P. hypsinotus.
Dorsal spines less than 15 (8 to 12). (8)
Antennal scale very narrow, distal half of blade narrower than the thickness of the adjacent spine, P. stenolepis.
Antennal scale of moderate width, distal half of blade wider than the thickness of the adjacent spine, P. danae.
Pandalus montagui Leach is the type species of the genus Pandalus. About 15 species are now placed in this genus. The western Atlantic form of Pandalus montagui has been described as a distinct species Pandalus levigatus Stimpson, but is currently synonymized with the typical eastern Atlantic form. In the North Pacific a distinct subspecies Pandalus montagui tridens Rathbun, 1902, is usually recognized.
Pandalus montagui tridens Rathbun, 1902, Proc.U.S.Nat. Mus. 24, 901. Type locality: Off North Head, Akutan Island, Alaska, 132 m. Range: Bering Sea south to California and the Kuriles.
Pandalus montagui montagui Leach, 1814. Range: Arctic Atlantic south to British Isles and Massachusetts.
United Kingdom: The pink shrimp
Norway: Spraglete raeke (or reke)
Denmark: Rejekongen
Germany: Felsengarnele
Newfoundland: The striped pink shrimp
Vernacular names
United Kingdom: Prawn (The Wash)
Sprawn (Morecambe Bay)
Shank (Northwest England)
The species has been described in detail by Calman (1899).
Land areas:1 212 British Columbia (Butler, 1964); 214 Ontario (Whiteaves, 1901); 215 Quebec (Whiteaves, 1901); 216 Newfoundland and Labrador, St. Pierre and Miquelon (Squires, 1957); 217 New Brunswick, Nova Scotia, Prince Edward I. (Squires, 1957); 250 Greenland (Stephensen, 1935); 511 Denmark (Bjorck, 1913); 513 Iceland (Wollebaek, 1900); 514 Norway (Norman, 1894); 515 Svalbard Bear I, Jan Mayen (Grieg, 1926); 516 Sweden (Jagersten, 1936); 521 Netherlands (Wollebaek, 1900); 522 Belgium (Wollebaek, 1900); 531 Ireland (Kemp, 1910); 532 United Kingdom (Calman, 1899); 534 Scotland (Orkneys, Shetlands, Hebrides) (Kemp, 1910); 535 Northern Ireland (Kemp, 1910); 716 Soviet Is. in Arctic Ocean and Spitsbergen (Novaya Zemlya, Franz Josef Land, Severnaya Zemlya, Novosibirs Kiye, Ostrova) (Greig, 1926).
Sea areas:1
ANE (Atlantic, N.E.) North limit 78°N, South limit 50°N; ANW (Atlantic, N.W.) North limit 65°N, South limit 41°N; INE (Pacific, N.E.) North limit 65°N, South limit 39°N.
The main features of the area of distribution appear to be low temperature, and moderate to high salinity.
Eggs are carried externally by the females, attached to the pleopods, mainly during the period November to March, while the adults are in the offshore part of their range (Mistakidis, 1957).
Larvae are liberated mainly during the spring and the summer (April to October), while the adults are in the more inshore part of their total range. (Lebour, 1940, 1947; Allen, 1963).
Adults are found in estuaries, coastal waters, fjords and in the open sea. The depth range is approximately 2 to 3 fm (3.7 to 5.5 m) to approximately 400 fm (732 m). (Rathbun, 1904; de Man, 1920; Grieg, 1926; Mistakidis, 1957; Squires, 1961; Allen, 1963).
P. montagui is more common in shallow water, where, in depths between 10 and 50 fm (18.3 and 91.5 m) it may support commercial fisheries.
There is a pronounced movement into estuaries and shallow waters in the spring and movement back into deeper water in about October. In southeast England a small proportion remains in shallow water throughout the winter months (Mistakidis, 1957).
P. montagui tends to occur in the shallower water, with P. borealis replacing it at depths greater than approximately 50 fm (91.5 m). (Wigley, 1960; Allen, 1963). Very considerable fluctuations in commercial catches have been noted to occur from day to day (Mistakidis, 1957), and from year to year.
Temperature range
Adults are found in waters of temperature range -1°C to 21°C (Allen, 1963; Mistakidis, 1957; Squires, 1957, 1961). However, this species is widespread at the lower, rather than the higher temperatures within this range. On the American Atlantic coast, all records are from water below 10°C (Wigley, 1960).
Substratum
Adults are found in areas with substrata of sand, mud, gravel and rock, but many observations indicate that abundance is often correlated with a hard, rather than a soft bottom. They are rarely abundant in extensive muddy areas, or in the bottoms of fjords and basins. (Mistakidis, 1957; Grieg, 1926).
Depth
See section 2.22.
Food
Small crustaceans, bivalve molluscs, and encrusting animals are all eaten, together with tubiculous worms such as Sabellaria and Pectinaria (Mistakidis, 1957).
Salinity
P. montagui occurs in water of salinity ranging from 25‰ to 35‰, but is most usually found in water of salinity between 32‰ and 34‰.
1 Land and sea areas as given by Holthuis and Rosa (1965).
Hermaphroditism
Populations of P. montagui contain varying proportions of protandrous hermaphrodites. Up to 50 percent of O-group individuals start life as males and subsequently change sex to become secondary females. The remainder of the population mature and continue through life as primary females (Mistakidis, 1957; Allen 1963).
On the Canadian Atlantic seaboard transitional stages have been noted among catches of P. montagui, but no primary females were found (Squires, 1965). No primary females have been observed among catches of P. montagui tridens on the Canadian Pacific seaboard (Butler, 1964).
Sexual dimorphism
The secondary sexual characters of the genus Pandalus appear on the endopodites of the 1st and 2nd pairs of pleopods. In the male the endopodite of the 1st pleopod bears a copulatory organ and the endopodite of the 2nd pleopod an appendix masculina. The latter structure is absent in the female and the endopodite of the 1st pleopod is lanceolate in shape (Mistakidis, 1957). These structures can be distinguished in young individuals of 5.0 mm to 6.0 mm carapace length, but below 4.6 mm the copulatory organ is not easily distinguished and the whole appearance of the endopodite is not unlike that of a female individual (Mistakidis, 1957).
The detailed development of the copulatory organ and the appendix masculina has been described by Mistakidis (1957). Both these organs are fully developed at copulation. After mating, which usually occurs in autumn, these organs undergo atrophy and the female condition is approached (Mistakidis, 1957).
The development of the female involves elongation of the distal end of the endopodite of the 1st pleopod which eventually becomes lanceolate in shape. The appendix interna associated with the endopodite of the 2nd pleopod gradually enlarges but it is always adjacent to the endopodite at its base. The ovigerous setae appear before oviposition and disappear with the first moulting after the hatching of larvae (Mistakidis, 1957).
Generally the males are smaller and narrower in body, while the females are broader and possess larger epimera. The latter become deeper before oviposition and protect the eggs and the abdominal segments (Mistakidis, 1957).
In the female the genital orifices are present on the inner side of the coxopodite of the 3rd pair of pereiopods and in the male on the coxopodites of the 5th pair of pereiopods.
On the east coast of England mature males are found when about 7 mo old (in November) at a carapace length of 9 to 11 mm. The first sign of transformation from male to female is found in February. Transitional stages occur throughout the summer months becoming less and less numerous by the end of December and January. A small percentage of individuals in the active male stage occurs throughout the year, indicating that they might function twice as males before undergoing change to the female condition. The ovaries begin to mature in early August and the majority of females reach maturity by November-December.
Mating is believed to be promiscuous.
Fertilization is external and occurs at the time of laying eggs.
Mistakidis (1957) found a direct correlation between carapace length and the number of eggs carried by female pink shrimps (Fig. 2): the smallest number of eggs carried was 136 (carapace length 7.2 mm) while the largest was 3,796 (carapace length 16.0 mm).
There is no substantial variation in the number of eggs carried at the beginning, middle and end of the breeding season (Mistakidis, 1957).
Allen (1963) calculated that the number of larvae hatched out inshore exceeds the number hatched offshore by 1.5 times; but stated that many of the larvae produced by the deep-water population were lost either by predation or current drift.
The parasitic isopod, Hemiarthrus abdominalis, was found to cause up to a 50 percent decrease in the number of eggs carried (Mistakidis, 1957).
Number of spawnings per year
Each female lays one batch of eggs during the breeding season (Allen, 1963).
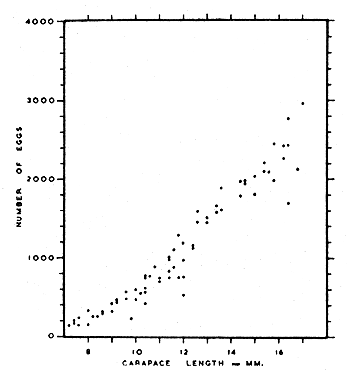
Fig. 2 The relationship between size and fecundity in Pandalus montagui. (from Mistakidis, 1957)
Spawning seasons
In the North Sea, egg laying commences at the beginning of November and continues until January or February with a maximum of ovigerous females in December (Mistakidis, 1957).
Sequence of spawning of individuals in a population
The larger females in the population lay their eggs first, followed by the females which spawn for the first time in their lives (Mistakidis, 1957).
Relation of the time of breeding to that of related species
The time of breeding of P. montagui and the rate of development of the ova follow a pattern similar to that of P. borealis.
Egg laying in the Northumberland population of P. borealis occurs between the beginning of October and the end of November, the larvae hatching by the middle of April (Allen, 1959).
Location and type of spawning ground
In southeast England, concentrations of ovigerous females are found in areas of deep water. One of the wintering grounds was located off the suffolk coast at depths between 30 and 40 fm (55 and 73 m) (Mistakidis, 1957)
Ratio and distribution of sexes on spawning grounds
On the offshore wintering grounds off the Suffolk coast, England, 74.00 to 97.50 percent of the population were found to be females, of which 95 to 97 percent were ovigerous (Mistakidis, 1957).
Eggs may be spherical or slightly ovoid in shape and measure, at laying, 0.6 to 0.8 mm. Initial colour is bright emerald green, changing, as the eggs develop, to light green with a slight violet tint. They are attached by a cement-like substance to the ovigerous setae which are developed on the pleopods before oviposition.
Mistakidis (1957) observed that eggs laid in November showed no appreciable growth during the first three months of incubation. By February a considerable proportion showed an increase in size, mainly those exhibiting late gastrula and ‘early’ eye-pigmentation stage. During April and May the majority of eggs were between 1.00 mm and 1.20 mm in length, all being in ‘early’ or ‘late’ eye-pigmentation stage. Hatching probably takes place shortly after the ‘late’ eye-pigmentation stage is reached (Mistakidis, 1957).
Generally eggs above 0.88 to 0.90 mm in length are in the ‘early’ eye-pigmentation stage and eggs above 0.98 mm in length in the ‘late’ eye-pigmentation stage. In the latter stage the eyes of the embryo measure 0.12 to 0.20 mm, and the yolk does not occupy more than 1/5 to ¼ of the volume of the egg (Mistakidis, 1957).
Newly hatched larvae have a length between 2.4 and 3.4 mm. Under laboratory conditions larvae have been reared to the megalopa stage through 11 zoeal stages at 10°C, but only to stage 5 at 18°C. Zoea larvae collected from the plankton in the Firth of Clyde and the Irish Sea showed six stages of development.
The approximate lengths of zoea larvae at successive stages are as follows (measurements taken from the tip of the rostrum to the tip of the telson):
| Stage: | I | II | III | IV | V | VI |
| Length: (mm) | 3.6 | 4.3 | 5.2 | 6.5 | 8.3 | 9.8 |
Planktonic specimens have red or orange chromatophores on each antennular peduncle and antennal scale, on the anterior face of each eye stalk, and on the posterior face after stage II, on the mouthparts, maxillipeds, pereiopods, the junction of the 6th abdominal somite and the telson. A large chromatophore, usually predominantly yellow, is always present beneath the central carapace, with 2 smaller orange chromatophores just in front.
The rostrum bears both dorsal and ventral teeth in the last stage (Pike and Williamson, 1964).
Little is known of the early adolescent phase. Immature O-group individuals with carapace length of 5 mm to 9 mm, have been recorded in mid-July by Mistakidis (1957) and Allen (1963).
Average life expectancy is 3 to 4 yr. Individuals of carapace length between 16 mm and 19 mm are considered to be 4 or 5 yr old.
When pink shrimps were exposed to air for 30 min their survival rates varied between 1.5 and 15 percent at temperatures between 10°C and 17°C. The survival rates were found to vary with temperature and weather conditions (Mistakidis, 1958).
Gadus merlangus and Gadus luscus are known to be major predators, but other species of fish such as Pleuronectes sp., Raja sp., Solea sp., and Cottus sp., which occur very frequently on the fishing grounds of southeast England (Mistakidis, 1958) are also believed to be predators.
In eastern Canadian waters P. montagui is eaten by cod in Newfoundland, Grand Banks and Labrador (Mikhalkovich, 1964). Squires (1957) observed the presence of P. montagui in the stomachs of cod and of seals. Fifty specimens have been recorded in one seal stomach in eastern England (Sergeant, 1951). Tuck and Squires (1955) refer to a colony of guillemots in Ungava Bay, Canada, feeding on limited numbers of pink shrimps.
The external parasite Hemiarthrus abdominalis (Krøyer) has been noted on P. montagui caught off Greenland, Norway and Britain (Allen, 1966). In most populations, the percentage of parasitized individuals is low. Mistakidis (1957) records infection of less than 3 percent and, Allen (1963) of 2 percent, of pink shrimp populations of eastern England.
Mistakidis (1957) stated that it was not unusual to observe variations in the composition of stomach contents from individuals feeding over different and widespread grounds. He found that concentrations of pink shrimps often occurred in areas inhabited by Sabellaria sp. Occasionally complete heads of Sabellaria were seen in the stomach contents which seemed to suggest that “P. montagui crawls over the Sabellaria colony, cutting off the protruding heads of the sedentary worms”.
Mistakidis (1957) observed some paucity in feeding before egg laying; but Allen (1963) stated that even during the last week or so before egg laying, when the ova are occupying almost all the available space, P. montagui continues to feed. It seems likely that the only time that pink shrimps do not feed is when the mouth parts are soft following a moult (Allen, 1963).
P. montagui occurring off the southeast coast of England are found to feed mainly on polychaetes of which Sabellaria spinulosa comprises 30 to 50 percent (Mistakidis, 1957). The polychaete fraction of the stomach contents of shrimps caught off the Northumberland coast is dominated by Pectinaria (Allen, 1963).
Crustaceans, foraminiferans, hydroids and fish scales are also frequently found in the stomachs of pink shrimps (Mistakidis, 1957; Allen, 1963).
In the laboratory, Mistakidis (1957) found that the meat of Mytilus edulis and Crepidula fornicata was readily eaten by P. montagui.
Growth of both sexes is rapid during the first year, becoming much less during the next 2 or 3 years (Mistakidis, 1957; Allen, 1963).
By November the O-group males off north-east England reach a mean carapace length of approximately 10.0 mm, the females having a mean carapace length of 11.5 mm. This disparity between the males and females is maintained throughout their lives and is due to the fact that the males mature 3 to 4 weeks before the females (Allen, 1963).
1-group males not having undergone a sex change after the first breeding season attain a mean carapace length of 14 mm by their second winter and males surviving to the third year attain a mean carapace length of approximately 17 mm (Allen, 1963).
Mistakidis (1957) showed that populations of P. montagui occurring off the southeast coast of England are migratory, appearing in shallow water in early spring and largely, although not completely, disappearing in late autumn. He concluded that the offshore emigration is related to low temperature and breeding.
Allen (1963) found no evidence of a massive inshore migration in Northumberland waters. Migrations appeared to be restricted to the offshore movement of 1-group and 2-group shrimps. Males that do not change sex migrate to deeper water during March when they mature a second time as males. Many of the females also migrate to deeper water, but this takes place during October (Allen, 1963). Mistakidis (1957) also reported ill-defined summer migrations which he related to high temperatures and feeding.
Murie (1903) and Kemp (1910) described P. montagui as being gregarious and migratory. No precise information on the gregarious habits of the species is available but rapid decreases in catches at certain times of the year indicate either mass movements to another area or scattering of the population.
In the first year 30 to 50 percent of the year-class mature as primary females (Jagersten, 1936), the remainder as males. In the second year primary females continue to function as females. Some mature males continue as active males, but a large majority of the male population changes sex to become secondary females. In the third and possibly subsequent years, almost all individuals in the population function as females (Mistakidis, 1957; Allen, 1963).
Sex ratio of the catch
There is invariably a predominance of females taken in the catch. The proportion of males taken is dependant largely on mesh size.
Sex ratio on spawning grounds
See section 3.16.
Allen (1963) found that the population of P. montagui off the Northumberland coast is composed predominantly of O-group males and females and smaller numbers of 1-group females while that in 50 fm is composed predominantly of 1- and 2-group males and females. This difference can be explained in terms of sex change and migration. (Table I).
The age distribution of the catches and the age of shrimps at first capture are dependent on mesh size. However, shrimps are generally caught first at an age of 3 to 4 mo.
Age at maturity
Males and females mature at 7 to 9 mo (Mistakidis, 1957; Allen, 1963).
Allen (1963) found a difference in the size composition of populations of P. montagui in shallow and deep waters off the Northumberland coast (Fig. 3). These differences can be explained in terms of sex change and migration (see also 4.12).
Fox and Wingfield (1937) noted a difference in size of populations of P. montagui at Plymouth and Kristineberg. The maximum total length of the Thames population (90 mm) contrasts with a single specimen measuring 160 mm from a depth of 100 m off the west coast of Norway (Wollebaek, 1908; Mistakidis, 1957). This difference in size may, in addition to depth, be correlated with low temperature in high latitudes, as was found in P. borealis (Allen, 1959).
The length at first capture is dependant to a certain extent on mesh size. Fishing trials during 1955 showed that the smallest specimens were caught during July at the time of the appearance of the new year-group. Nets of mesh size ranging from 14.5 to 22.5 mm (between opposite knots of a stretched mesh) caught shrimps of carapace length of approximately 4 mm (Mistakidis, 1958).
Catches are riddled to separate the commercial and non-commercial sizes of shrimps and the latter returned to the sea. The riddle most commonly used in the Thames area has a mesh size of approximately 5.2 mm. Mistakidis (1958) showed that riddling did not separate all the non-commercial shrimps from the catch, and that a large proportion will perish if the period between hauling and riddling the catch is more than 20 min.
There is considerable variation in the maximum size reached by P. montagui over its range of distribution. Maximum sizes (overall length from tip of rostrum to tip of telson) recorded for the species in various areas are as follows:
P. montagui
| North Sea | 160 mm | (Balss, 1926) |
| Newfoundland | 130 mm | (Squires, 1961) |
| Vancouver | 90 mm | (Butler, 1964) |
| Southeast England | 90 mm | (Mistakidis, 1957) |
| Ungava Bay | 108 mm | (Squires, 1957) |
| Maine | 110 mm | (Scattergood, 1952) |
| Norwegian coast | 160 mm | (Wollebaek, 1900) |
| Labrador | 130 mm | (Squires, 1957) |
P. montagui tridens
North Pacific coast 110 mm (Rathbun, 1904)
The relation between carapace length and total weight for shrimps occurring off the southeast coast of England is shown in Fig. 4.
Measurements of total length and carapace length show that growth is isometric. For both the sexes and the transitionals total length can be derived from the carapace length by the multiplication factor 4.5 for shrimps from the southeast coast of England (Mistakidis, 1957), and 4.26 for shrimps occurring in Northumberland waters (Allen, 1963).
In the shrimp fishery of southeast England an average trawl tow of one hour takes a catch of between 32 and 70 l. Catches vary with the time of year, state of tide and on the fishing area. Catches of four times this quantity are not unusual and catches of 450 l/h have been known (Mistakidis, 1957).
TABLE I
Average percentage age-group composition of shallow and deep-water populations of Pandalus montagui off the Northumberland coast for the years 1954–1961 (after Allen, 1963)
| Shallow (20–30 fm) | Deep (50 fm) | |||||
| Gp 0 | Gp 1 | Gp 2 | Gp 0 | Gp 1 | Gp 2 | |
| yearly average | ||||||
| a | 76.4 | 23.4 | 0.2 | 9.7 | 80.8 | 9.5 |
| b | 71.0 | 28.7 | 0.3 | 9.3 | 72.0 | 18.7 |
| c | 44.5 | 59.0 | 100 | 42.7 | 38.9 | 85.0 |
| Average for breeding period, November-May | ||||||
| a | 86.5 | 13.4 | 0.1 | 36.2 | 63.2 | 0.6 |
| b | 76.0 | 23.8 | 0.2 | 27.4 | 71.6 | 1.0 |
| c | 46.8 | 96.1 | 100 | 41.1 | 61.4 | 100 |
a Total population b Female population
c Percentage of females present in each year group
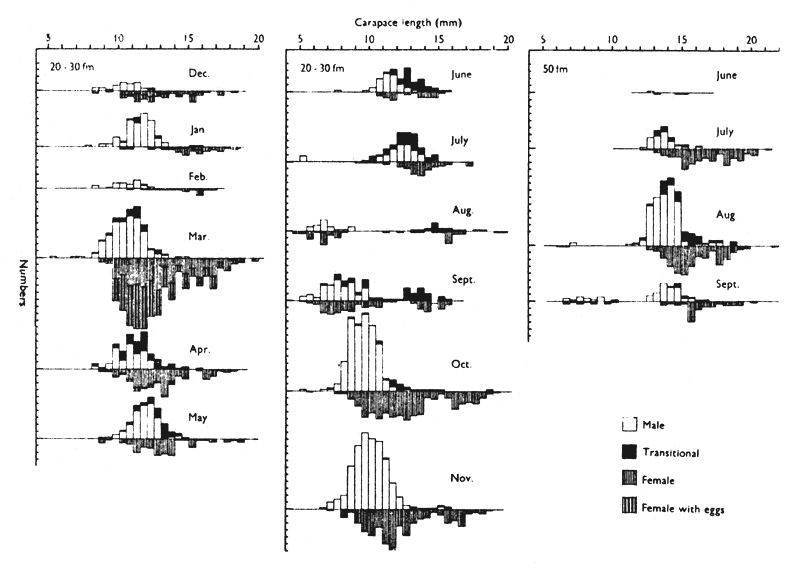
Fig. 3 Population histograms showing sex changes in the shallow-water population over a full year and including, for comparison, records from the deep-water population for the months June to September. (from Allen, 1963)

Fig. 4 The relationship between carapace length and body weight in Pandalus montagui, males and females from various areas in southeast England.
Variations with depth
Though P. montagui occurs in depths between 2 and 400 fm (3.7 to 732 m), commercial fisheries normally occur in areas of depths between 10 and 50 fm (18.3 to 91.4 m).
Seasonal variations in available stock
See ‘Migrations’ (3.51).
Annual egg production rates
Allen (1963) calculated that there were 1.5 times as many larvae released in shallow water (20 to 30 fm (36.6 to 55 m)) as compared with deep water (50 fm (91.4 m)) off the Northumberland coast, but that seven times as many O-group adults occurred in shallow water as compared with deep water. The most probable explanation is that deep-water larvae are eaten by planktotrophic adult P. borealis, the dominant species of the deep-water natant community, although currents may carry deep-water larvae inshore. Zoeae of P. montagui have been found in the stomach contents of P. borealis by Allen (1963). (See also 3.15).
There is no precise information on the variation in annual recruitment but large variations over a number of years have been experienced in southeast England.
See section 3.34.
There is no indication that fishing is a major source of mortality in any fishery for this species.
It would appear that P. montagui is more susceptible to chemical pollution than most other species (Portmann, personal communication).
In the United Kingdom, although some use is made of small otter trawls, a majority of boats fish for P. montagui with beam trawls. The length of the beam is normally between 18 and 25 ft (5.5 to 7.6 m) and may be of wood or metal tubing. Trawl heads are ‘D’ shaped and of mild steel construction. Ground ropes may have a chain, wire or rope core, covered by rubber discs, wooden rollers, or possibly wrapped with several layers of old rope, to a diameter of 3 to 10 in (7.6 to 25.4 cm), depending on the nature of the ground to be worked (Mistakidis, 1958).
Trawls are invariably of nylon or courlene. In the Thames area a mesh size between 19 mm and 22 mm is generally employed. Trawls are protected beneath by rubber or netting chafers, and sometimes buoyed by corks on the upper surface. The early large beam trawls have been replaced by smaller, lighter gear and recent developments have tended towards multiple beam trawl rigs, towed by one vessel.
Echo sounding gear is of limited application, being of value only in the identification of substrates and the avoidance of submarine obstructions.
Trawling is carried out by 30 to 45 ft (9.1 to 13.7 m) decked boats, powered by diesel engines of 30 to 50 hp and equipped with boilers for cooking the catch. There has been no significant change in the type of boats employed in recent years.
The main fishing areas in the United Kingdom are the Wash (east coast), the south-east coast (including the Thames estuary), Morecambe Bay and the Solway Firth.
No fisheries outside the United Kingdom are known to the authors.
Commercial fisheries occur in coastal waters in depths between 10 and 50 fm (18.3 to 91.4 m) and are generally associated with estuaries.
In southeast England fishing is carried on between April and October, or possibly to December, depending on weather conditions.
No attempt has been made to measure fishing effort and so no data are available.
Changes in mesh size and their effects
From data collected from fishing trials with shrimp nets of varying mesh, Mistakidis (1958) found that satisfactory catches of commercial-size shrimps were obtained with nets having meshes of 21 and 23 mm in the codend. Nets with small mesh retained higher percentages of non-commercial shrimps and yielded bigger catches, while nets with large mesh retained smaller percentage of non-commercial shrimps but with a corresponding decrease in the size of the catch.
Total annual yields
Statistics relating to catches of shrimps in the United Kingdom include both P. montagui and Crangon crangon, the brown shrimp. Annual yields for both species together fluctuate between 900,000 and 3,700,000 kg. An estimate of the proportion of each species making up the total catch is difficult since different proportions are caught in different areas. Mistakidis (1957) estimated that 80 percent of the total shrimp catch in the Thames area was P. montagui, while in the Morecambe Bay fishery P. montagui accounts for as little as 5 percent of the total catch.
In the United Kingdom the Sea Fisheries Committees have the power to introduce byelaws relating to shrimp fishing. These regulations govern the dimensions and mesh size of the net, duration of the haul, mesh size of the riddle, minimum depth of water for the return of the riddlings and the close season. The bye-laws enforced by the various Sea Fisheries Committees are summarized in Table II.
TABLE II
Sea Fisheries Committees bye-laws relating to shrimps (Pandalus montagui and Crangon vulgaris)
| Max. width of net | Max. length of net | Minimum mesh (wet) | Maximum duration of haul | Riddle min. size of meshes | Minimum depth of water for return of riddlings | Miscellaneous | |
| Northumberland | - | - | - | - | - | - | - |
| North Eastern | - | - | - | 1/2 hr | - | - | - |
| Eastern | - | - | - | - | - | - | - |
| Kent and Essex | - | - | 108 rows of knots per yd 144 " " " " " 8 ft from cod end | - | - | - | - |
| Sussex | 25 ft headline | - | - | 1/2 hr | - | 6 in | - |
| Southern | - | - | - | - | - | - | - |
| Devon | - | - | - | 1 hr | - | 6 in | - |
| Cornwall | - | - | - | - | - | - | - |
| South Wales | - | - | 7/8 in width 3/32 in flat gauge | - | - | - | - |
| Lancs. and Western | 30 ft headline or beam | 1 ½ times beam or headline | 5/8 in width 3/32 in flat gauge | - | 3/16 in × 3 in | 1 ft | Close season 1 Dec. - 30 April |
| Cumberland | - | - | Square gauge width 3/8 in sides | - | - | - | - |
Allen, J.A., 1959 On the biology of Pandalus borealis Kroyer, with reference to a population off the Northumberland coast. J.mar.biol.Ass.U.K., 38(1):189–220
Allen, J.A., 1963 Observations on the biology of Pandalus montagui. (Crustacea: Decapoda) J.mar.biol.Ass.U.K., 43(3):665–82
Allen, J.A., 1966 Notes on the relationship of the bopyrid parasite Hemiarthus abdominalis (Krøyer) with its hosts. Crustaceana, 10(1):1–6
Balss, H., 1926 Macrura der Deutschen Tiefsee - Expedition, 2. Natantia, Teil A. Wiss. Ergebn.dt.Tiefsse-Exped. ‘Valdivia’, 20:217–315
Bjorck, W., 1913 Biologisch - Faunistische Untersuchungen aus dem cresund. I. Pantopoda, Mysidacea, Decapoda. Acta Univ.lund., Afd.2, 9(17)
Butler, T.H., 1964 Growth, reproduction, and distribution of Pandalid shrimps in British Columbia. J.Fish.Res.Bd Can., 21(6):1403–52
Calman, W.T., 1899 On the British Pandalidae. Ann.Mag.nat.Hist., 7(3):27–39
de Man, J.G., 1920 Decapoda of the Siboga expedition. (IV) Siboga Exped., 39a3:318 p.
Fox, H.M. and C.A. Wingfield, 1937 The activity and metabolism of poikilothermal animals in different latitudes. 2. Proc.zool.Soc.Lond.,(A) 107:275–82
Grieg, J.A., 1926 Decapod crustacea from the west coast of Norway, and in the North Atlantic. Bergens Mus.Årb., 7:1–53
Hemming, F., 1965 Substitution on the official list of generic names in Zoology of a revised entry relating to the generic name Pandalus Leach, 1815. Opin.Decl.int.Commn Zool.Nom., 1:D (D.10):243–54
Holthuis, L.B., 1950 Decapoda (K IX) A. Natantia, Macrura Reptantia, Anomura en Stomatopoda (K X). In Boschma, H., Fauna van Nederland, 15:1–166
Holthuis, L.B., 1955 The recent genera of the caridean and stenopodidean shrimps, (Crustacea, Decapoda, Natantia), with keys for their determination. Zool.Verh., Leiden, 26:1–157
Holthuis, L.B. and H. Rosa, 1965 List of species of shrimps and prawns of economic value. FAO Fish.tech.Pap., (52):21 p.
Jagersten, G., 1936 Uber die Geschlechtsverhaltnisse und das Wachstum bei Pandalus. Ark. Zool., 28A (20):1–26
Kemp, S., 1910 The Decapoda Natantia of the coasts of Ireland. Scient.Invest.Fish.Brch.Ire., 1:3–190
Kinahan, J.R., 1858 On the occurrence of a new Irish Aesop prawn in Dublin Bay. Proc.nat. Hist.Soc.Dublin, 2:79
Leach, W.E., 1814 Original description of Pandalus montagui. In Brewster's Edinburgh Encyclopaedia (Crustaceology) 7:432–36
Leach, W.E., 1815 Pandalus, Pandalus annulicornis descriptions. Malac.Podophth.Brit., plate 40(2)
Lebour, M.V., 1940 The larvae of the Pandalidae. J.mar.biol.Ass.U.K., 24:239–52
Lebour, M.V., 1947 Inshore plankton of Plymouth. J.mar.biol.Ass.U.K., 26:527–47
Mikhalkovich, V.I., 1964 On the distribution of shrimps in the Labrador and Newfoundland areas. Mater.rybokho.Issled.severn.Bass., (11):59–62
Mistakidis, M.N., 1957 The biology of Pandalus montagui (Leach). Fishery Invest., Lond., (2), 21(4):1–52
Mistakidis, M.N., 1958 Comparative fishing trials with shrimp nets. Fishery Invest., Lond., (2), 22(1):1–22
Murie, J., 1903 Report on the sea fisheries and fishing industries of the Thames Estuary. I. London
Norman, A.M., 1894 A month on the Trondshjeni Fjord. Ann.Mag.nat.Hist., 13(6)
Ortmann, A.E., 1893 Decapoden und Schizopoden. Ergebn.Plankton-Exped., 2 (Gb):1–120
Pike, R.B. and D.I. Williamson, 1964 The larvae of some species of Pandalidae (Decapoda) Crustaceana, 5(4):265–84
Rathbun, M.J., 1902 Descriptions of new Decapod Crustaceans from the west coast of North America. Proc.U.S.nat.Mus., 24:888–905
Rathbun, M.J., 1904 Decapod crustaceans of the northwest coast of North America. In Crustaceans, Harriman Alaska Expedition, 10: 3–211. Reprinted 1910. Publs.Smithson.Instn., (1997):1–337
Rathbun, M.J., 1929 Canadian Atlantic fauna, 10 Arthropoda. Publ.biol.Bd Can., (38)
Sars, G.O., 1882 Oversigt of Norges crustaceer. Forth. VidenskSelsk.Krist., (18)
Scattergood, L.W., 1952 The northern shrimp fishery of Maine. Comml.Fish.Rev., 14(1):1–15
Sergeant, D.E., 1951 The status of the common seal (Phoca vitulina, L.) on the East Anglian coast. J.mar.biol.Ass.U.K., 29:707–17
Squires, H.J., 1957 Decapod crustacea of the Calanus expeditions in Ungava Bay, 1947–1950. Can.J.Zool., 35, (Calanus Ser., 11):463–93
Squires, H.J., 1961 Shrimp survey in the Newfoundland fishing area (1957–1958). Bull.Fish.Res. Bd Can., (129):29 p.
Squires, H.J., 1965 Decapod crustaceans of Newfoundland Labrador, and the Canadian Eastern Arctic. Manusc.Rep.Ser.(biol.)Fish.Res.Bd Can., (810)
Stephensen, K., 1935 The Godthaab expedition, 1928. Crustacea decapoda. Medd.Grønland, 80(1):1–94
Stimpson, M., 1860 Crustacea Macrura. Proc.Acad.nat.Sci.Philad., 12:22–47
Tuck, L.M. and H.J. Squires, 1955 Food and feeding habits of Brunnich's murre (Urialomvia lomuia) on Akpatok Island. J.Fish.Res.Bd Can., 12(5):781–92
Whiteaves, J.F., 1901 Catalogue of marine invertebrata in eastern Canada. Geological Survey of Canada, Ottawa, 275 p.
Wigley, R.L., 1960 Note on the distribution of Pandalidae in New England waters. Ecology, 41(3):564–70
Wollebaek, A., 1900 Decapoda collected during the fishery investigations carried out under the leadership of Dr. Hjort in 1897–1898. Rep.Norw.Fishery mar.Invest., 1(4):1–29
Wollebaek, A., 1908 Remarks on decapod crustaceans of the N. Atlantic and the Norwegian fjords. Bergens Mus.Arb., 1908(12):74 p.