Hatchery facilities
The sophistication of rearing facilities required in hatcheries will depend to a large extent on the method of management of the seed production system. If seed are harvested with 6 mm mesh seine nets, for example, only fry above 1–2 g will be harvested. On the other hand, if smaller meshed nets (e.g., mosquito netting) are used, or harvesting frequency is increased to once every one to two weeks and all broodfish are inspected, seed collected will contain eggs at various stages of development: hatchlings, pre-swim-up and swim-up fry. Harvesting at 5-day intervals will result in only egg collection. To maintain the higher yields from frequent harvesting, the eggs, hatchlings and early fry need to be incubated artificially.
Hatcheries need to be equipped with high quality water, suitable incubation systems and two to three sizes of tanks to nurture the seed to a size suitable for stocking. The efficient management of these facilities is crucial since poor care of the seed will drastically reduce overall fry production.
In addition, these facilities may also be required in some hatcheries which normally strip broodstock of their gametes to produce specific hybrids.
Technology for artificial rearing of tilapia seed
Manual stripping and fertilization of eggs. Healthy sexually active broodfish are an important prerequisite for successful stripping of gametes.
(a) Males
The quantity and quality of milt varies between males, the greater their social dominance, the greater the quantity of milt and therefore the ease of stripping. To strip a male the abdomen is squeezed gently from the pectoral area towards the genital region. This procedure also empties the bladder of urine and care should be taken not to mix it with the milt.
It is often more difficult to obtain milt from Tilapia species than from mouth-brooders. If sufficient milt is not available males can be sacrificed and their testes removed and macerated on a dry slide. Prior to fertilization the macerated testes should be mixed with about 0.5 ml of water or 0.9% saline solution and then added to the eggs. To ensure sperm activity, sperm from at least three males should be pooled and used.
(b) Females
A female can only be successfully stripped if all her eggs have been ovulated into her ovarian cavity. When the eggs have ovulated, the ovipositor descends and is almost perpendicular to the body. The genital papilla generally becomes swollen and pinkish in colour. Often the body coloration deepens and females become more aggressive. Females showing these external characteristics are ready for manual stripping.
(c) Procedure for stripping and fertilization of eggs
Females should be caught gently in a deep net and wrapped in a damp paper towel or cloth to reduce skin damage and drying. If large broodfish are used they may be sedated to facilitate handling. If anaesthetics are used the anaesthetized fish should be rinsed in freshwater before stripping to avoid the drug contaminating the eggs.
The eggs of mouth-brooders and substrate spawners need to be treated differently. In mouth-brooders the genital area of the fish should be wiped and eggs gently squeezed from the ovary into a dry clean container. To expel the eggs, the abdomen should be squeezed from the pectoral region diagonally down towards the genital area. Care must be exercised not to press too hard as this would rupture the ovarian blood vessels. Should this happen the fish should be returned to the holding tank. If an attempt is made to strip an unovulated female the ovarian blood vessels may rupture and result in large intra-ovarian blood clots which will hinder or prevent future spawnings.
The eggs are then fertilized with milt from the desired male(s). The ovarian fluid is an excellent sperm activator. Therefore, provided sufficient milt is available (0.1 ml is adequate for up to 1 000 eggs), undiluted milt should be added directly to the eggs, stirred gently and left for 3–5 minutes. The fertilized eggs are then washed with clean, warm water and transferred into incubators.
With substrate spawners, their sticky eggs should be stripped onto a clean, dry, flat surface, e.g., perspex slide, glass, etc., and spread gently into a layer one egg deep with a brush or feather. The milt of the desired males is then spread over the eggs and allowed to stand for five minutes. The eggs should then be gently rinsed with clean water to wash off excess milt. The slides containing the eggs can then be easily incubated.
Egg development. As for all fishes, the rate of development of eggs and fry of tilapias is temperature-dependent (Tables 10.7 and 10.8).
Incubation of tilapia eggs. The method required to rear tilapia eggs varies between species. In mouthbrooders the most important requirement is that eggs should be kept in gentle motion, whereas Tilapia eggs develop in static conditions. All eggs, however, should be reared in well aerated, clean water of high quality.
(a) Incubation of eggs of substrate spawners
As already mentioned, the sticky eggs of Tilapia spp. are stripped onto slides. The slides are immersed in a container of well-aerated water.
(b) Incubation of eggs taken from brooders
Irrespective of the type of container used, it is preferable to use a system that is unlikely to fail, i.e., use gravity flow, minimize use of pumps, etc. Ideally, containers should be made from locally available materials. The commonly used systems are described below.
Conical or funnel up-welling jars (or “Zug” jars). These types of containers, used widely in carp propagation, are the most commonly available and therefore used in most hatcheries.
These incubators can be made from glass, plastic, perspex, fibreglass, metal or linen. Glass jars of 5–15 1 can be specially blown but, like perspex, are very expensive and may not be available. Cloth or metal incubators are cheaper to construct but it is not possible to visually inspect the egg or fry mass.
In these incubators water flows in from the bottom of the container; the flow can be adjusted to suspend the egg or fry mass in continuous motion in the water column.
Round-bottomed, down-welling containers. These containers were originally called MacDonald jars and were made of glass. They are cylindrical in shape with a round bottom. Water enters the container from a fixed pipe from above. The flow can be adjusted to gently rotate the eggs.
Table 10.6
RATE OF DEVELOPMENT OF HATCHERY REARED OREOCHROMIS SPP. EGGS
AND FRY ARTIFICIALLY INCUBATED AT 28°C
| Stage of development | Age | |
| Hours | degree days | |
| 2 cells | 1.5–2 | 1.8–2.3 |
| 4 cells | 2–3 | 2.3–3.5 |
| 8 cells | 3–4 | 3.5–4.7 |
| 16 cells | 4–5 | 4.7–5.8 |
| 32 cells | 5–6 | 5.8–7.0 |
| Blastula | 10–12 | 11.7–14.0 |
| Embryonic shield-closure of blastopore | 14–36 | 16.3–42 |
| Hatching | 90–102 | 105–119 |
| Early fry (swim-up) | 120–144 | 140–168 |
| End of yolk-sac | 216–432 | 252–504 |
Large range due to variation in egg size. The larger the egg the longer the time to end of yolk-sac stage.
Table 10.7
HATCHING TIMES OF ARTIFICIALLY INCUBATED O. NILOTICUS
EGGS AT VARIOUS TEMPERATURES
| Temperature (°C) | Time to hatching | |
| (days) | (degree days) | |
| 24 | 5–6 | 120–144 |
| 28 | 4 | 112 |
| 30 | 3 | 90 |
| 34 | 2 | 68 |
An alternative cheap source of either funnel or round-bottomed containers is plastic soft-drink bottles. The tops or bottoms can be easily cut off to make ideal incubators.
In both of the above systems bad eggs are automatically flushed away with the outflow.
(c) Requirements of developing eggs and fry
High water quality and husbandry standards are essential for healthy development. Developing eggs and fry require oxygen-rich water, especially when large quantities of seed are being incubated. Attempts should be made to maintain oxygen levels in excess of 4–5 mg/l. To this end, plankton and algae-free water should be used. Although the plant material oxygenates the water during the day it rapidly consumes oxygen at night.
The maintenance of optimal rearing temperature is essential. Temperatures of below 24°C and above 35°C will lead to high egg mortalities, and any hatchlings may also be very weak and eventually die. Tilapia eggs and fry should be reared between 25° and 30°C, but optimal growth occurs between 28° and 30°C.
During development, large quantities of ammonia and carbon dioxide may be produced. These metabolites need to be flushed away by maintaining a continuous flow of water. If ammonia levels are allowed to exceed 5 mg/l the growth of the fry may be inhibited and the gills of developing fry may be damaged.
The maintenance of correct pH is also important to ensure healthy development, and should be kept between pH 6.5 and 7.5. A pH of below 4.5 or above 8.5 will result in high egg and fry mortalities. A high pH in combination with low water hardness may result in weakened egg shells and consequently prematurely hatched and weak fry.
It is common to treat Tilapia eggs with chemicals during incubation to control bacterial or fungal infections, using one of the following treatments:
| Disinfectant | Dosage (mg/l) |
| Formalin | 1 000–2 000 |
| Malachite green | 1–5 |
| Acriflavin | 750–1 500 |
| Buffodine | 50–100 |
Hatching and rearing of larvae
The eggs of substrate spawners hatch within 48 h at 28°C, while mouth-brooded eggs hatch within 96 h.
The larvae of all tilapia species have bulky yolks; unable to swim, they will sink. They do not have functional mouths or gills and rely on their superficial blood vessels on the tail and body for their oxygen supply.
The larvae of mouth-brooding species should be reared in the incubators until they become free-swimming. In up-welling and down-welling incubation systems, the swim-up fry can be separated automatically if the outflow is channelled into the rearing tank thus minimizing handling.
Incubation of the sticky eggs of substrate spawners on slides helps to separate the larvae from the egg shells and from bad eggs. Prior to hatching, the slide should be turned such that eggs are on the underside of the slide. This allows the hatching larvae to fall away from the slide.
Timing of initial feeding
The correct timing of initial feeding of developing larvae is crucial for ensuring high survival and producing high quality fry. Under hatching conditions, the species of tilapia and rearing temperature are two major factors affecting the transition to exogenous feeding.
The larvae of Tilapia spp. which produce smaller eggs than mouth-brooders, develop faster and are able to ingest food by four days after hatching at 28°C and three days at 30°C.
In mouth-brooders, exogenous feeding occurs at a later age and is also temperature-dependent. At 24°C onset of feeding occurs at about eight days after hatching (12–13 days post-spawning), while at 30°C feeding initiates at four days (8–9 days post-spawning).
The onset of feeding in tilapia larvae coincides with swim-bladder inflation. Therefore it would be a prudent policy to monitor rearing temperature and to begin initial feeding of larvae just before they become early fry. It should be realized that at this stage even though the fry still possess yolk reserves these are inadequate to meet all the growth demands. Therefore if initial feeding is delayed even by a day, the growth of fry may be sub-optimal and the fry will be small for their age. These fry may also be weakened and easily become victims of disease and cannibalism.
In view of the above, the quality of seed may also be related to the seed production system. Larvae of Tilapia spp. are not orally reared and therefore the quality may not be affected by parental behaviour. Among mouth-brooders, however, larvae are capable of exogenous feeding before the time at which they are normally initially released from the buccal cavity. In addition, if the brooder delays the release of her clutch, e.g., under high stocking densities, or releases the fry for only short periods for feeding, they may begin to lose condition. Thus if naturally reared fry are collected from hapas, tanks or other confined production systems, they may be sub-optimal for their age. Under these conditions, fry from young brooders may be further disadvantaged because of their smaller yolk reserves.
Therefore one method for the fry producer to maximize the quality of fry from mouth-brooders is to remove or encourage the release of larvae from brooders prior to the early-fry stage for hatchery rearing. Larvae that approach the early-fry stage can then be fed continuously from this point onwards.
Rearing of early fry
Tanks are ideal for nurturing early fry. In these carefully controlled environments their feeding can be closely monitored, health checked, and predators kept out to ensure high survival. With proper care, survival of fry up to 2–3 g can exceed 90%.
Circular (1–2 m diameter) or rectangular (area 1–16 m2) tanks may be made of cement, fibreglass, metal or plastic. Such tanks are easily managed by one or two persons. To encourage early feeding the water depth for the initial week should be kept to about 15 cm and then gradually increased. To remove metabolic by-products, faeces and uneaten food, a good water flow is necessary. Any remaining food and faeces should be removed daily.
If complete artificial diets are fed, early fry may be stocked at up to 2 500/m2 for the first week. The advanced fry will then need to be graded and stocking density gradually decreased to about 1 500/m2. With careful management up to 30 000, 2–3 g advanced fry can be produced in a 16 m2 tank. These fry are then transferred to fingerling grow-out facilities.
Under semi-intensive conditions where green water systems are used and feeding is supplemented with low protein diets (ab. 25%), lower fry densities must be used. Under these conditions and tanks should be stocked with 100–200 early-fry/m2.
If green water systems are used, tanks sould be fertilized with chicken manure (100–150 kg/ha/day) and phosphates and nitrates (10 kg/ha/day). These rates may need to be adjusted, the main objective being to achieve visibility down to a maximum of 30 cm. Hatchery operators using green water systems should be aware of oxygen depletion due to algal respiration especially before sunrise. Dissolved oxygen levels should never be allowed to fall below 2 mg/l.
All-male seed production. A widespread problem in tilapia culture is early sexual maturation and breeding of fish in the grow-out phase. This phenomenon results in unwanted seed production and overcrowding. Under these conditions food becomes limited, resulting in the production of small fish (50–100 g) which may command a lower market price. Also, the diversion of energy from growth to egg production leads to a further decline in growth. Thus there may be large differences in the average size of males, which may be at least 50% larger.
To prevent overcrowding, and to allow for better management, all-male seed-stock may be used. These are usually produced by one of three methods: hand-sexing and mechanical grading; hybridization; androgenic sex reversal (Table 10.8).
(a) Hand-sexing and mechanical grading of fingerlings. In mouth-brooders, the sexes of mature fish may be determined by the examination of the genital papillae (Fig. 10.3). Generally, the sexes can be distinguished when fingerlings are about 30–35 g, but sexing is considerably easier in fish weighing more than 50 g. Depending on experience, about 2 000 fingerlings may be sexed in a day. Mechanical graders have also been used to separate the larger males from the females but this is less reliable than hand-sexing.
Table 10.8
COMPARISON OF THE SUITABILITY OF VARIOUS METHODS
FOR ALL-MALE SEED PRODUCTION
| Methods | Suitability | |
| Advantages | Disadvantages | |
| Hand sexing
| Requires no special equipment or facilities | Labour intensive; wastage, as females are discarded; not 100% reliable due to human error |
| Mechanical grading | Cheap and quicker than hand sexing | Poorer sex separation than hand sexing |
| Hybridization | No wastage of fry as all are used Hybrid growth vigour and hardiness | Need to obtain and maintain pure species Greater management of broodstock. All-male progeny in broods not consistent. Low fry production due to species incompatability |
| Androgenic sex reversal | Consistent all-male seed production, if conducted correctly. No wastage of fry. No need for pure species | Availability and added cost of synthetic hormone and organic solvents. High level of technical skill and management required |
(b) Hybridization. Even though various workers have shown that more than 25 different hybrid combinations produced over 80% male seed, the use of hybridization for commercial all-male seed production is limited. Of the various hybrid combinations only a few consistently produce all-male progeny. Oreochromis hornorum is the only known species which consistently produces all-male fry when crossed with O. niloticus or O. mossambicus. The O. niloticus and O. hornorum cross is preferred because of its superior growth performance. Other crosses between O. niloticus and O. aureus which produce between 80 and 90% males are also used. This cross combines the growth vigour of O. niloticus with the cold-tolerance of O. aureus to produce a hybrid population that is largely male and is cold-tolerant.
(c) Androgenic sex reversal. It shows greatest promise and is now increasingly used. The objective of this technique is to convert genetic females into “males” by feeding sexually undifferentiated fry on diets mixed with androgenic hormones. Thus at the end of the treatment the seed will contain genetic males (normal) and genetic females sex-reversed into “males”. The success of the sex reversal treatment, however, will be dictated by factors such as quantity of hormone-treated diet ingested, dosage, age of fry used for treatment and method of diet preparation.
The most commonly used synthetic androgenic hormone is 17 methyltestosterone (17∞ MT). Various doses ranging from 30 μg/g diet to 60 μg/g diet have been used.
For most effective results, a diet containing 40 μg 17∞ MT/g diet is fed for 40 days from first-feeding. If the duration of feeding is reduced, however, or if fry older than one week are used, then the percentage of males may be reduced.
Diets and feeding routines for tilapia fry
During early development, the metabolic activity and growth of fry is very high. Therefore, to sustain optimal growth, the hatchery operator should ensure that the food available to fry contains sufficient quantities of protein for tissue construction and is nutritionally well-balanced.
Natural foods. If ponds and outdoor tanks are used to rear fry, a wide selection of natural foods may be available to the fish. Their diets consist largely of a mixture of algae, bacteria and detritus. By adding organic and inorganic fertilizers to the water, the quantity and range of food items can be increased. Addition of organic manures introduces particulate matter which is rapidly broken down by bacteria, protozoans and small invertebrates and encourages the growth of algae, phytoplankton and zooplankton all of which form an excellent source of protein and add to the balance of the diet.
Natural foods also play an important role in green water culture systems using supplementary diets. Under this management system, some natural food is available to the fry at all times, providing essential vitamins and minerals which may be deficient in the supplementary diets.
As the stocking density of fry is increased, however, the quantity of natural food available to each fry will decrease and therefore the quantity and quality of supplementary or formulated diets will become more important.
Complete diets. Correctly formulated diets containing required amounts of proteins, lipids, amino acids, fatty acids, minerals and vitamins are crucial if healthy and strong fry are to be produced in the absence of natural foods.
Protein is of primary importance for fish growth, providing the basic materials for tissue-building and energy. To sustain the high metabolic rates of the rapidly growing fry, protein requirements are very high. The quantity of protein that is ultimately included in the diet will depend on the age of the fry and the source and quality of protein.
For tilapias the protein requirement declines with age (Table 10.9). For rapidly growing tilapia fry, protein levels of between 30 and 50% have given good growth. For fry up to 0.5 g, 50% protein is required if fed 3–4 times/day at 10–15% of body weight/day. Lower protein levels may be adequate if feeding frequency is increased to eight times/day and ration increased to 20–25% of body weight/day. Diets containing protein levels of 30–35% have proved adequate when fed at 10–15% body weight/day in semi-intensive systems where natural food supplements the artificial diet.
Table 10.9
SUMMARY OF THE MAJOR NUTRIENT REQUIREMENTS OF TILAPIAS
(in percentage of dry diet)
| Nutrient | Size range of fish | |||
| up to 0.5 g | 0.5–10 g | 10–35 g | > 35 g | |
| Crude protein | 50 | 35–40 | 30–35 | 25–30 |
| Crude lipid | 10 | 10 | 6–10 | 6–8 |
| Digestible carbohydrate | 25 | 25 | 25 | 25 |
| Fibre | 8 | 8 | 8–10 | 8–10 |
Lipids are an important sources of essential fatty acids and of energy, twice that of protein. In addition, by providing lipid in the diet the protein may be spared for tissue construction. Lipid requirements are said to decrease with the age of the fry. For fry up to 10 g, lipid levels of 10% are recommended. For fingerlings, 6% is adequate (Table 10.9).
Carbohydrates may be included in the diet as an energy source to spare protein (Table 10.9). Sources such as rice, barley, oats, millets and sorghum contain 50–60% carbohydrates. They are a cheap source of energy and are included as a bulking agent and as a binder.
Vitamins and minerals can be obtained as premixes. Vitamin premix should be used at approximately 2% of the complete dry diet. If natural food is available, the level may be reduced.
Feeding routine. Under natural conditions, tilapias are omnivorous and choose to browse on detritus, phyto- and zooplankton, algae and small invertebrates of appropriate size. They are equipped with simple stomachless guts and as such feed continuously during daylight hours.
Under hatchery conditions where formulated diets are fed, the growth and survival of tilapia fry will be influenced by factors such as particle size, feeding frequency/ration, and feeding method.
Particle size of food. The particle size acceptable to first-feeders depends on the size of the fry. Table 10.10 summarizes suggested particle sizes for tilapia fry.
Table 10.10
PARTICLE SIZE OF DIET ACCEPTABLE TO TILAPIA FRY
|
| Fish size | Particle size (mm) | |
| Weight (g) | Standard length (cm) | ||
| Tilapia spp. early fry | 0.003–0.005 | 0.3–0.4 | 0.1–0.2 |
| Mouth-brooders early fry | 0.006–0.015 | 0.5–0.9 | 0.2–0.3 |
| Fry of all species | 0.05–0.25 | 1–2 | 0.25–0.5 |
| 0.25–0.5 | 2–3 | 0.5–0.75 | |
| 0.5–1 | 3–4 | 0.75–1 | |
| 1–3 | 4–5 | 1–2 | |
| 3–30 | 5–9 | 2–3 | |
Feeding frequency and ration. First-feeders grow best when they are fed continuously. They are equipped with a simple gut that is designed to cope with small quantities of food.
Under hatchery conditions feeding frequency will be determined by the management of the rearing tanks. If green water systems are used, a feeding frequency of 3–4 times a day is adequate. If flow-through systems, high stocking density or clear water systems are used, first-feeders should be fed 6–8 times a day.
Food intake (measured as percent body weight/day) is inversely related to fish size. The ration given to fry under hatchery conditions will also depend on the availability of natural food. In green water systems, rations of between 10 and 15% body weight/day appear adequate.
In intensive culture conditions, the rations need to be higher, generally between 25 and 30% body weight/day. As fish grow the ration should be reduced. When fry reach 1–2 g they should be fed at 10–15% body weight/day, and by 30 g at 3–5% body weight/day.
Feeding at the correct frequency and ration is important for both survival and growth. Under low rations and feeding frequencies, size variations of individual fish will increase considerably. Under these conditions, cannibalism of small fry by larger individuals may be as high as 30–35%. Under-fed tilapia fry become very aggressive. In the case of O. mossambicus it has been shown that starved 20–30 mm fry can easily kill and consume fry up to half their own size.
The feeding frequency and ration may also be related to water temperature and oxygen stress. As the oxygen level and water temperature decrease, or fish become stressed by disease, transportation, etc., food consumption will decrease. Under these conditions feeding ration will need to be reduced. Conversely, at higher temperatures, feeding should be increased. Feed may be delivered to tilapias by hand, or by use of demand or automatic feeders.
Pond culture
Pond culture is the oldest and still the most widely practised method of growing tilapias. During the 1940s and 1950s, many thousands of ponds were built across Africa. The method of culture, in which ponds were stocked once and then sequentially netted leaving the smaller fish behind to grow and spawn, resulted in an extended culture period and heavy spawning. The size of fish and the yield at harvest continued to fall until the farmer was so discouraged that he abandoned his ponds.
Today pond culture of tilapias has once again become popular, not only in Africa and Asia but also in the southern USA, thanks to changes in practices. Modern pond culture involves stocking grow-out ponds with fry, culturing over a fixed time period, draining the pond and carrying out a complete harvest. The range of methods encompasses both intensive and less intensive approaches.
Site selection and pond construction. Little will be said here concerning site selection and pond construction, it being assumed that detailed information is available elsewhere. Engineering considerations are of course the same for tilapia ponds as for ponds intended for culture of other species of fish.
Earth ponds are best constructed in sunny, open areas with gentle (0.5–2.5%) slopes. If the site has clay soils, then the pond will retain water better. A supply of water close by, sufficient to at least counteract losses through seepage and evaporation, is also essential. Concrete-lined ponds are prohibitively expensive and difficult to manage except in intensive operations (e.g., in Taiwan).
In theory, production ponds may be of any size or shape. Ponds of at least 200 m2 are recommended, although in Israel 8–10 ha ponds are not uncommon. Size will ultimately depend upon land and construction costs, time taken to fill the ponds with water, and management and marketing strategies. Where there are no topographical limitations, ponds should be square or rectangular in shape. The pond must be drainable, and to facilitate this the bottom should be sloped with the shallow end at the inlet and the deep end at the outlet. Pond depths of 1–1.5 m are ideal in most situations, although it may be necessary to dig deeper ponds if rainwater is the sole or prime source of water. A screened water source to prevent an influx of wild tilapia is essential.
Pond preparation and stocking. Before the newly constructed pond is filled with water it may be necessary to adjust the pH of the soils. Alkaline soils are rare and do not usually cause problems. However, acid soils are common in many parts of the world, and if the pH of the soil is less than pH 6 liming should be carried out.
Following liming, the pond should be filled with water and fertilized if necessary. Several days should elapse before the fish are introduced. Fish about 3 cm long should be used to stock grow-out ponds.
Extensive pond culture. In extensive systems, the productivity of the pond depends solely upon soil and water chemistry (and thus the available plant nutrients), and location (sunlight, temperature). These factors limit the amount of primary production which takes place in the ponds, and this in turn controls the rest of the pond system. There is thus a good correlation between primary production and fish yield from extensively managed tilapia ponds.
Yields, however, are low and indeed are similar to natural water bodies with similar rates of primary production (50–300 kg/ha/year). Typical stocking densities are between 500 and 2 000 fry/ha. Not surprisingly, this is not a common method of rearing fish and economic studies suggest that it would be difficult to recoup capital costs of pond construction.
Semi-intensive pond culture. Production per unit area of pond can be increased through increasing stocking densities and food availability. Since primary production and fish production are correlated, one way to achieve this is to fertilize the ponds, thus encouraging planktonic algae to grow.
Inorganic fertilizers are normally prohibitively expensive. Moreover, as fertilization rates increase, they become increasingly less cost-effective; doubling the application rate of fertilizers does not double fish production. The reason seems to be that eventually there is so much alga in the pond that self-shading occurs (i.e., algal production becomes light-limited).
A more cost-effective method of pond fertilization is to use organic fertilizers. It is thought that they work by not only providing nutrients for algal growth, but also as a source of dead and decaying plant-based material (detritus) which is both a direct and indirect source of food for the fish. Particles are ingested directly by the fish but also provide a substrate for bacteria which in turn are fed upon by protozoa, zooplankton, benthic invertebrates and, of course, the tilapia. It is thus important that the manure forms particles as small as possible in the water to facilitate these processes.
Organic manures have proved effective even at high stocking densities. Typical stocking densities are 10 000–30 000 fry/ha. The quantity of manure that should be added depends upon moisture content and the biomass of fish in the pond. The manure should preferably be added regularly and frequently.
Water deoxygenation should be avoided. A good guide as to whether manuring has been effective is that the pond water should be a deep green, blue-green, or brown colour and visibility, measured by a Secchi disc, should be about 30 cm.
Although less effective than animal manures, simple compost made from various plant material can also be used. Bamboo or wooden cribs, approximately 2 m2, are built at intervals around the pond (approximately 1–2 cribs/100 m2 pond area) and these kept full with grass, vegetable wastes, kitchen wastes.
Using animal manures alone, tilapia production of 3–6 t/ha/year is possible. However, production may be further increased (up to 10 t) through the addition of supplementary feeds and these may also be used as an alternative to manuring. Since the protein level of natural foods in the ponds is high, carbohydrate-based materials are recommended. Any material will suffice; waste food, duck-weed, chopped grass, rice bran, etc.
Fish should be fed at least once per day. However, it is impossible to give hard and fast rules regarding feeding rate since it is dependent upon so many variables - fish size, stocking density, pond productivity, water quality, temperature, and feed quality. As a guide, food should be added slowly to the pond until no more is consumed by the fish.
Intensive culture in ponds. Intensive pond culture of tilapias is rare and is practised principally where there are water shortages or restricted growing seasons and where market prices are sufficiently high. Sometimes earth ponds are managed in this way. However, in Taiwan, Israel and Japan ponds of the type shown in Figure 10.8, are used. The walls, 1.5 m high, are built of brick or concrete and the bottom is of mud. Water level is maintained at around 1 m. Paddlewheel aerators constantly stir the water, creating a gentle, circular flow around the central outlet. Wastes concentrate in the centre of the pond and these are removed twice each day by dropping the pond level by 2.5 cm.
The ponds are around 2 000–2 500 m2 each and are stocked at a rate of 25–40 male fish/m2 at 30–50 g. They are fed three times per day on high protein (25–30%) pellets at a rate of 2% body weight per day. Feed conversion rates are consistently less than 2:1. Under favourable conditions, fish reach 600 g in 4–5 months at the higher densities and 1 kg in 5–6 months at the lower density.
Intensive pond production can give yields of 20–25 t/pond/ production cycle - equivalent to 200 t/ha/year. However, production costs are high and sophisticated technology using artificial aeration, formulated feeds and monosex male fish is necessary.
Tank culture
Other land-based systems for ongrowing tilapias include tanks and raceways. Both types of systems are used almost exclusively for commercial, intensive culture of tilapias and rely on high stocking densities, high protein feeds and, recycling systems excepted, the utilization of comparatively large volumes of water. In view of the high capital and operating costs, these systems are principally used where high market prices are in operation (e.g., production for export to European and North American markets, live fish restaurant trade, etc.) in countries such as Taiwan, Japan, Israel, Western Europe (Belgium, Netherlands), southern USA and in certain areas of Africa (Zambia, Kenya).
Tank design. Tanks of various materials, sizes and designs have been used to culture tilapias. In selecting materials, costs, durability and a smooth interior surface to reduce the risks of abrasion damage are important. Simple, inexpensive tanks may be readily constructed from sections of large diameter (1–2 m) concrete pipe set into a concrete base or from sheets of corrugated galvanized metal riveted and glued together, set into a concrete base and painted or coated with epoxy resin to give a less abrasive finish. Concretelined brick-built tanks are comparatively cheap to install. Fibreglass tanks are superior but are much more expensive.
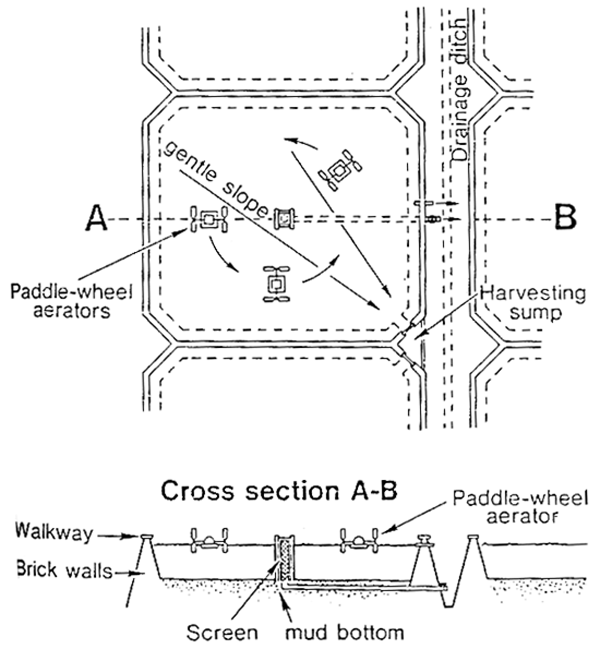
Fig. 10.8 Ponds for intensive culture of tilapia
Although a little more expensive, round tanks are far superior to square or rectangular tanks in terms of utilization of water, flow pattern, waste removal and in fish distribution. Oval tanks are also excellent but are expensive and complex to construct. Silos, which are large, deep tanks with a centralized inflow, are inexpensive to construct but are costly in terms of water use and maintenance. Stocks are difficult to observe, feed conversions are poor and there may be a wide range in sizes at harvesting. To date they have not been used for tilapias and are rarely used for aquaculture purposes outside the USA.
Tank size is usually determined by economic and management considerations. Although small tanks may be chosen as a result of availability of construction materials (e.g., pre-fabricated sections of concrete pipe), generally the larger the tank the lower the costs per unit volume. To ensure good cleaning efficiency, a tank diameter:depth ratio of 5–10:1 is recommended for large tanks. Tanks of greater than 10 m diameter are not recommended as they become increasingly difficult to manage. Tank layout should be determined by available space and topography (e.g., location of supply water, slope, shelter, etc.).
The water to the tanks may be supplied by gravity, or pumped, and may come from any source provided it is free from toxic pollutants. If water is in short supply some form of recirculation may be necessary.
Stocking and management of tanks. For intensive, grow-out tanks, larger (5–25 g) all-male fry are often stocked. As stocking density is increased, individual growth rate falls, although total production (kg/m3/month) may continue to increase up to a certain limit. There is thus a range of strategies that could be adopted by a farmer; to aim for high production of small fish, to opt for comparatively lower production of large fish, or to choose some intermediate strategy. Decisions should ultimately be based upon market prices.
The water supply to the tanks must serve two purposes: to supply DO for respiration and to remove waste feed and metabolites. As a rule of thumb, a supply of 0.5–1 litre water/kg fish/min is usually adequate, although this assumes reasonable temperatures (about 30°C), oxygen saturation of inlet water and adequate current velocities within the tanks. The latter is particularly important: if above 25–30 cm/sec, the fish must expend excessive energy to maintain station, whilst, if below 7.5–10 cm/sec, the wastes settle out in the tank and adversely affect water quality. Some form of additional aeration may also be necessary to help maintain adequate DO levels.
Good quality diets containing more than 25% protein should be used and comparable conversion rates to ponds and raceways usually occur. Tanks should be kept clean and excess food not allowed to accumulate. Growth should be monitored by regular sample weighings.
At the end of the production cycle (3–6 months), harvesting is a comparatively simple affair. The water level is dropped and fish removed using hand nets. Very high per-unit-volume production figures are widely reported for intensive tilapia tank culture operations, up to 6 000 t/ha/year. However, more typical production levels, as obtained in Kenya, Israel, USA and Taiwan, are around 5–10 kg/m3/month (1 000–1 200 t/ha/year). Whilst these figures may appear very attractive, it must be borne in mind that the data are principally from pilot-scale units, that the systems rely heavily upon high technology and operating costs, and that the economics have yet to be fully evaluated.
Raceway culture
Raceway design. Raceways are large, elongated tanks, although, because of their size, water flow characteristics and consequently fish distribution and water quality are much better. Raceways are usually constructed from reinforced concrete or bricks and cement and have an inflow at one end and an outflow or overflow at the other. The design is critical. Ideally water should have a laminar flow and approach “plug” flow conditions in which the entire water column moves at the same velocity, thus eliminating dead spots and short circuiting, and maintaining a high cleaning efficiency. In practice it may be necessary to compromise slightly, as excessive velocities may cause crowding of fish at one end. Length:width:depth ratios should be in the order of 30:3:1. The inlet should cover the entire width of the raceway and baffles may also have to be introduced to reduce turbulence.
Stocking and management of raceways. Raceways are not commonly used for tilapia culture. From available published data (USA, Kenya) initial stocking densities of 1–5 kg/m3 are used and production values of up to 13 kg/m3/month, using high protein feeds, have been achieved. Water exchange rates of at least three volumes per hour are recommended. One farmer in the USA has also grown tilapia in raceways below intensive channel catfish raceways and has claimed that, for every 2 kg of catfish, 1 kg of tilapia is also produced without any additional inputs.
Water-based culture systems
Cages, pens and enclosures are all water-based rearing systems. As the terms are often used interchangeably, it is necessary to define how they will be used here. Cages are totally enclosed on all, or all but the top sides, by mesh or netting, whereas in pen and enclosure culture the bottom is formed by the lake bed. Enclosures differ from pens in that they are formed from an enclosed natural bay. The shoreline forms all but one side, which is typically closed off by a solid, net or mesh barrier. Cages also tend to be smaller and are better suited to more intensive culture methods than enclosures or pens.
Cage culture of tilapias. Cages have a number of advantages over other rearing systems; they are comparatively inexpensive to construct and make use of existing water bodies, thus allowing non land-owning sectors of the community access to fish farming and providing a free supply of planktonic food for the caged fish. Management and harvesting are also simple, and the problems often associated with tilapia rearing (i.e., reproduction, over-recruitment, stunting) do not occur as the eggs or fry when released simply fall through the mesh floor of the cage bag. Cage culture of tilapias has become a major industry in the Philippines and is rapidly gaining in popularity in other parts of the world.
Expensive, high technology cages, such as those used for salmon production in Europe with steel or high density plastic collars are not necessary for growing tilapias, as inland water sites are less hostile than marine. Simple cages, built from locally available materials, are usually sufficient.
Two types of cages may be used, fixed or floating. The former are simple and cheap to construct, although they are generally smaller, are restricted to depths of less than 8 m or so and require soft-bottomed substrates so that the support posts may be installed without difficulty. They are thus not suited to reservoirs with large draw-downs and, because they are less strong than floating designs, require a more sheltered position.
The length of each side of a fixed cage should be 0.3–0.6 m longer than the dimensions of the bag. Almost any type of readily available material will suffice for the support posts - bamboo is ideal. Mature, seasoned culms are selected, cut to length (allow 1 m for anchoring in substrate, and water depth, + 2–3 m for superstructure) and trimmed to remove sharp projections using a machette. Holes are knocked in the submerged sections to facilitate installation. Corner poles are established first and buried to a depth of at least 0.6 m in the lake bottom. Using guide ropes stretched along each side, the remaining posts are then installed at approximately 1 m intervals. A row of laterals, formed from the tops of bamboos, is lashed to the uprights thus strengthening the structure and providing a walkway of sorts. Additional bracing may be used if necessary (Fig. 10.9a).
The net bag (see below) should have ropes (rigging) at 1–1.15 m intervals to facilitate attachment to the bamboo frame. At least 1 m of free-board netting is required to prevent fish jumping out.
Floating cages are more expensive to build but can be installed in deeper water and at more exposed sites. In theory the collar should be designed to withstand prevailing static (weight of bag, fish biomass, worker) and dynamic (wind, wave, current) forces.
The simplest floating cage collar may be manufactured entirely from rope and bamboo (Fig. 10.9b). The 5 m base sections from 20 bamboo culms are used for the four sides, the middle 1 m sections for the uprights and corner bracings, whilst the top 5 m sections are employed as handrails. All joints, including corners, are secured using nothing more than rope and bamboo pegs. The resultant structure has sufficient floatation properties to support a 4 × 4 × 4 m bag and one worker and has a serviceable working life in freshwater of 1.5–2 years.
Larger, more robust and more durable designs, with greater floatation capabilities, can be built using timber and oil drums (Fig. 10.9c).
Size of individual cages varies enormously, depending largely upon scale of operation, availability of construction materials, and design (see Table 10.11a).
Floating cages can be simply anchored in soft sediments using sandbag anchors (nylon bags filled with stones). The mooring line should be 3 times longer than the depth of water; the distance between the bottom of the cage bag and the lake bottom should preferably be at least 5 m.
Cage bags should be fabricated from knotless nylon netting. Top nets may also be fitted to deter predatory birds.
Site selection. Productive lakes or reservoirs, free from industrial effluents, are best for the majority (i.e., extensive or semi-intensive) of cage tilapia operations. Sheltered sites with adequate water depths should be selected. Poaching is often a problem with water-based aquaculture ventures and it may be necessary to choose sites which can be readily watched at all times. Sites with high densities of cages already established are likely to be poorer than less developed sites.
 | a. Fixed cages in Laguna de Bay, Philippines |
| b. Bamboo coller floating cage in Laguna de Bay, Philippines |  |
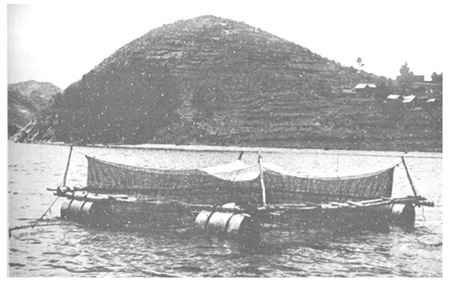 | c. Bamboo and oil drum floating cage in Lake Titicaca, Bolivia |
Fig. 10.9 Simple fixed and floating cages
Table 10.11
SUMMARY OF CAGE AND PEN CULTURE OF TILAPIAS
(a)
| Type | Common size | Shape | Capital costs (per m2) | Culture system | Comments |
| Fixed cage | 1–30 m3 | square/rectangular | low | ext./semi-int./int. | Production flexible; not suitable for exposed sites or water deeper than 8 m |
| Floating cage | 10–100 m3 | square/circular/rectangular | medium | ext./semi-int./int. | Production flexible; comparitively strong structure |
| Pen | 0.1–10 ha | variable | medium-low | ext./semi-int. | Inflexible production; not suitable for water deeper than 8 m; hard substrates can be a problem; good growth but poor recovery of stocked fish |
(b)
| Culture systems | Stocking density (fish/m3) | Food | Site | Average production (kg/m3/month) | Comments |
| Extensive | 4–10 | natural | lake, reservoir | 2 | Experience suggests it is difficult to sustain production due to over-exploitation of sites |
| Semi-intensive | 10–50 + | low protein (10%) plant and agricultural by-products | lake, reservoir, river | 4 | Most common method; addition of some food usually increases production unless bloom conditions prevail |
| Intensive | 50–200 + | high protein pellets (<25%) | lake, reservoir | 5–15 | Uncommon; advisable where growing season is short or where water resources are scarce; high market value for tilapias necessary |
Culture practices. In grow-out operations fish of 1–5 g are generally stocked, although bigger fish may be stocked if there is a short growing season or if market preferences are for larger fish. Cages are stocked in early morning or late afternoon when temperatures are lower.
Stocking densities are highly variable. For extensive culture, which is carried out in highly productive lakes, stocking densities of less than 10 fish/m3 are generally used, whilst at the opposite end of the spectrum, intensively-managed farms may stock at densities in excess of 200 fingerlings/ m3 (Table 10.11b). Stocking densities may also change with season as the availability of natural food changes.
As fish grow, the mesh size of the nets used should regularly increase (Table 10.12).
Table 10.12
RECOMMENDED MESH SIZES FOR TILAPIA CAGE CULTURE
| Size of fish | Purpose | Mesh size (side length mm) |
| Fry (12 g) | Nursery | 1–3 |
| Fingerling (12–30 g) | Grow-out | 4–8 |
| Grower (30–200 g) | Grow-out | 10–20 |
| Grower (200 + g) | Grow-out | 20–25 |
| Breeder (150 + g) | Breeding | 1–3 |
Most cage-based operations are semi-intensive and a variety of feedstuffs, principally low protein, high carbohydrate, plant-based materials are used. Feeding rates vary with the availability of natural food, the quantities and quality of supplementary food available and fish size. For example, a typical 77% rice bran, 23% trash fish mixture is usually fed at a rate of 3–5% body weight/day, the feed either being simply cast on the water surface or moistened and rolled into balls before feeding to help minimizing feed loss.
Typical yields from different types of tilapia cage culture operations are summarized in Table 10.11b.
Pen and enclosure culture. Pens and enclosures are not commonly used for tilapia culture although in the Philippines, where both systems are used for milkfish (Chanos chanos) culture, they have been employed to a limited extent.
Bamboo or wooden posts are driven into the lake bottom and a net is attached so that an area of the lake, sometimes as large as several square kilometres is effectively fenced off. A double net structure may sometimes be used for extra security.
Fish are typically stocked at rates of 12–50/m2 and, at higher densities, some supplemental feed may be used. Production from pens and enclosures, however, is poor, averaging less than 0.1 kg/m2/month, although growth rates are reportedly excellent. Harvesting is a problem; seine or gill nets are used with the result that only 10–30% of fish stocked are recovered.
Pens and enclosures are expensive to construct and can have negative social consequences, privatizing large areas of a common resource with resulting restrictions to fishing, access and navigation.
Tilapias in polyculture
Although tilapia are usually reared in monoculture, in some parts of the world they are grown in polyculture with other fish species in an effort to improve yields. Tilapias - principally Oreochromis species - are increasingly used in polyculture systems in combination with carps, freshwater prawns and catfishes. There is also some interest in culturing together several tilapia species which differ in feeding habit. A number of polyculture combinations is given in Table 10.13.
The ratios and densities of the species used in a particular polyculture combination are of prime importance. In theory, they should be determined from knowledge of the feeding habits of the fish species and the availability of different types of food in the pond; in practice, they are determined by trial and error. Other considerations, such as market price and management, may greatly affect how ponds are stocked. Ponds are most easily managed if, when harvested, all species have reached marketable size. In this way, intermediate harvesting with its attendant losses is avoided.
Although yields per unit area are improved through polyculture, there are a number of disadvantages. Reliable hatchery supplies of a number of species must be available. Moreover, sorting species during harvesting is expensive, requiring considerable extra manpower and, possibly, specialized grading equipment. The income generated from the high overall yields must cover these expenses, otherwise polyculture is not viable.
Integration of tilapia culture with agriculture
Integration of tilapia farming with agriculture is becoming increasingly important in countries where water or suitable feedstuffs are in short supply, and where the economics of rearing tilapias appear marginal.
Tilapia farming may be readily integrated with a number of both large and small-scale agricultural systems including livestock rearing (poultry, cattle, pigs, sheep, etc.), vegetable and cereal growing, mixed agriculture and irrigated farming.
Livestock wastes (faeces, urine, uneaten food) are excellent for fertilizing fish ponds, although it must be borne in mind that these wastes are also excellent for fertilizing crops and so may not be always available. Moreover, collection of wastes can be prohibitively time-consuming unless the animals are confined for at least part of the day, preferably close to or even above the fish ponds. As an approximate guide, Table 10.14 suggests the range of livestock numbers necessary for fertilizing tilapia ponds adequately.
Table 10.13
EXAMPLES OF VARIOUS POLYCULTURES INVOLVING TILAPIAS
(data from various sources)
| Country | Species | Stocking density (No./ha) | Comments |
| Israel | common carp (1) | 5 000 | Typical, commercial stocking densities. Departures from this, however, are common |
| mullet (2) | 2 000 | ||
| silver carp (3) | 300 | ||
| tilapia (4) | 10 000 | ||
| Israel | freshwater prawns (5) | 20 000 | Yields of freshwater prawns independent of fish densities densities |
| tilapia | 9 000 | ||
| grass carp (6) | 300 | ||
| silver carp | 1 000 | ||
| USA | channel catfish (7) | 4 940 | Experimental. Catfish production increased over control pond (no tilapia). At lower stocking densities polyculture is less successful |
| tilapia (8) | 1 400 | ||
| China (Guangdong) | silver carp | 750 | Just one of many different polycultures found throughout China. Many do not involve tilapias. |
| tilapia (9) | 4 000 | ||
| common carp | 375 | ||
| black carp (10) | 75 | ||
| Wuchang bream (11) | 525 |
(1) Cyprinus carpio;
(2) Mugil cephalus;
(3) Hypophthalmichthys molitrix;
(4) O. niloticus x O. aureus;
(5) Macrobrachium rosenbergii;
(6) Ctenopharyngodon idella;
(7) Ictalurus punctatus;
(8) O. mossambicus;
(9) O. niloticus;
(10) Myopharyngodon piceus;
(11) Megalobrama amblycephala
Table 10.14
LIVESTOCK NUMBERS RECOMMENDED FOR TILAPIA PONDS
(number/ha)
| low biomass/low yields | high biomass/high yields | |
| ducks | 500 | 5 000–10 000 |
| chickens | 1 000 | 7 000–10 000 |
| pigs | 15 | 150–200 |
| cattle | 20–40 | 200–300 |
Almost any wastes from cereal or vegetable crops can be added as supplementary feeds to ponds. However, the Chinese also grow a number of plants such as brassicas, sorghum and sweet potatoes, along pond dike walls for feeding to fish. Stocking with Tilapia species feeding on macrophytes would be necessary in this case.
In Israel, a semi-arid country where agriculture is largely irrigation-dependent, fish farming has had to become integrated with prevailing farming practices. Often water storage ponds may be used only for 4–5 months to rear a single crop of fish, the waste-rich water then being used during the dry period for irrigation purposes. Storage dams of 10–40 ha and 5–7 m deep are also used for carp/tilapia polyculture, the dams being constructed with terraces to facilitate routine sampling and harvesting. Yields (extrapolated) of 8–14 t/ha/year can be achieved.
Irrigation canals are also used for cage culture of tilapias in some parts of the Near East, China and Indonesia. Although this is sometimes highly successful, problems with fast or highly variable flow rates and increased sedimentation have also arisen. In Central Thailand, tilapias and other fishes are often released into orchard irrigation canals, to be recaptured using nets and traps at a later date.
Fish culture in rice fields, in which fish are either grown together or in alternation with a rice crop is practised in many countries, including China, USSR, Thailand and the Philippines. This can often be achieved with little change to traditional rice growing practices, resulting not only in a crop of fish for no extra labour, land or input costs, but also a reduction in weeds and pests. In the Philippines, tilapias are stocked at a rate of around 4 000/ha and yield crops of around 300 kg/ha in three months. However, problems can also arise, including damage to rice plants and restrictions in the types and quantities of pesticide that can be used.
Practical diets for on-growing tilapias
Tilapia culture may be defined as extensive, semi-intensive or intensive on the basis of feeding. In extensive culture, the fish must rely entirely, or almost entirely, on natural food items, although production of natural food may be stimulated through fertilization. In semi-intensive aquaculture, available natural food is supplemented by the addition of low protein (below 20%) materials, usually based on agricultural by-products or plant materials. In intensive aquaculture, all, or almost all of the nutritional requirements are met by the farmer. High protein (above 20%) feedstuffs, often involving animal or fishmeals, are used.
Diets for intensive culture. The aim in formulating diets for intensive tilapia culture is to produce a food which meets not only all the dietary requirements, but also promotes good growth, yet at a price which enables the farmer to sell his product at a profit. These aims may be contradictory since protein, the single most important item in the diet in terms of ensuring good growth, is also the most expensive. In formulating practical tilapia diets it may often be necessary to accept slightly inferior growth in order to keep feed costs within acceptable limits.
Choice of material should not only be based upon composition, but also upon palatability, availability, costs, storage life and the possible presence of any toxic compounds. Either plant or animal protein sources may be used. The most promising of the plant materials are the oilseed cakes, particularly soybean, cotton seed and sunflower seed. They are widely available and cheap, although often deficient in one or more essential amino acids. Moreover, several contain toxins. Amongst the legumes, ipil-ipil (Leucaena spp.) and beans contain relatively high levels of high quality protein although both also contain toxins and may require pre-treatment before feeding to tilapias.
Animal proteins such as meat meals, fish meals, meat and bone meals, blood meals, etc., are excellent sources of high quality proteins and are rarely deficient in any of the essential amino acids, although some are not particularly palatable. They are also extremely expensive and ideally should be used to make up dietary deficiencies in amino acids, vitamins and minerals rather than as a prime source of dietary protein.
Additional lipid may be required to make up for deficiencies in the diet, and plant or fish oils which contain high amounts of unsaturated fats should be used in preference to terrestial animal fats. Almost any readily available, plant-based carbohydrate source, such as rice bran, brewery wastes or wheat middlings can be used to bulk out the diet. Vitamin and mineral premixes should also be included.
In addition to ensuring that nutritional requirements are met, it may also be necessary to include additive to control oxidation and fungal growth and binders which help in pelleting feeds.
There are thus many different diet formulations which can successfully be used to intensively culture tilapias. It is recommended that wherever possible two or three plant protein sources are used and that at least 25% of the protein requirements are fulfilled by animal protein sources, thus minimizing the risk of amino acid and vitamin deficiencies. A number of specific diet formulations are given in Table 10.15. However, it is probably wise to consult nutrition specialists if considering a large-scale commercial intensive tilapia culture operation.
Table 10.15
SOME DIETS SUITABLE FOR INTENSIVE CULTURE OF TILAPIAS
(modified from Jauncey and Ross, 1982)
| Ingredients | Diet 1 (tilapia 0.5 g) | Diet 2 (tilapia 0.5–35 g) | Diet 3 (tilapia > 35 g) |
| Brown fishmeal | 30 | 10 | 5 |
| Hydrolysed feather meal | 15 | 5 | 3 |
| Meat meal | 5 | 5 | 5 |
| Soybean cake meal | 5 | 12 | 4 |
| Groundnut cake meal | 10 | 12 | 10 |
| Cotton seed cake meal | 5 | 20 | 20 |
| Rice bran | 12 | 22 | 39 |
| Dried distillers solubles | 10 | 10 | 10 |
| Vitamin premix | 1 | 1 | 1 |
| Mineral premix | 3 | 3 | 3 |
| Lipid supplement | 4 | - | - |
| Composition (% of diet) | |||
| Protein | 50 | 35 | 30 |
| Lipid | 11 | 9 | 8 |
Supplementary feeds for semi-intensive culture. A supply of supplementary feeds almost always results in improved production over extensive methods. Usually, low-protein, plant-based materials, such as rice bran and brewery wastes, are used. Choice of materials should depend upon cost (including transport) and availability as well as quality. It may also be necessary to switch from one food source to another during the production cycle as these factors change.
Feed preparation, storage and distribution
Intensive diets may be administered either in meal or pellet form, the latter being either moist or dry. In pond culture, there is some evidence that unpelleted diets may be just as satisfactory as pellets. However, in tanks where there is little access to natural feeds and because leaching of nutrients and vitamins from small particles is high, it is advisable to feed pellets. In cages, too, pellets are superior as they reduce losses. Moist pellets seem to give higher production results with tilapia than dry pellets, although they are difficult to store. Appropriate pellet sizes are given in Table 10.16.
Table 10.16
RECOMMENDED PELLET SIZE AND FEEDING RATES FOR
ON-GROWING TILAPIAS
(From several sources)
| Fish size (g) | Pellet size (mm) | Feeding rate (% body weight/day) |
| less than 20 | 1–2 | 6–4 |
| 20–100 | 2 | 3 |
| 100–250 | 3 | 3 |
| 250 + | 4 | 3 |
Supplementary feeds may be administered either dry in meal form or moistened and formed into shape.
Feeding rates vary with fish size and biomass and it is therefore important to keep good stock records. Ideally the feeding frequency for intensively cultured tilapias should be around six times per day for fry, although this can be reduced to three times per day for grow-out operations. Supplementary feeds are usually only offered once or twice daily. Feeding should, of course, be modified under adverse environmental conditions such as low dissolved oxygen and low temperatures.
Hand-feeding, although ideal, is time-consuming and for large farms it may be necessary to install automatic or demand feeders. Feedstuffs should be stored for as short a time as possible, in cool, dry, vermin-free conditions.
Tilapia farming, like other types of fish farming, is not without problems; disease, poor water quality and predation are frequently encountered by fish farmers, irrespective of the species being reared. Moreover, tilapias have a number of traits, such as maturation at an early age and small size when confined in ponds, which are undesirable in a farmed species. In the following section, a number of these problems and their solutions are briefly discussed.
Early maturation
Maturation, when only two or three months old and at a correspondingly small size (8–10 cm), is frequently reported by tilapia farmers. Fish then continue to breed at 4–6 week intervals with the result that they become stunted and populations increase dramatically. This problem is particularly acute with monoculture in earth ponds, but does not arise in net cages, where fertilized eggs and fry simply fall through the net floor of the cage.
There are two solutions to the above problem: prevention and cure. Stunting and overpopulation may be avoided by judicious choice of species or by the culture of fish of only one sex (monosex culture). In certain species, such as O. niloticus or O. aureus sexual maturation of the majority of the population occurs at a somewhat later age (5–6 months), even in crowded pond conditions, when most fish are around 150–200 g. However, these species may not always be readily available - at least as pure species - and market preferences may be for larger fishes.
Monosex culture of males is preferable to that of females, since the males grow considerably faster than the females. The simplest method of producing all-made seed (see section 10.7) is to sex the fish by examination of the urinogenital papillae and to discard the females. However, the consensus of opinion is that this can only be done with any certainty when the fish are 50 g or larger in size and although success rates of up to 97% have been claimed, in most circumstances success is only around 80%. Moreover, it is time-consuming (estimated rate is 2 000 males per worker per day), requires trained staff and results in the rejection of females.
The less satisfactory solution to overcrowding in ponds is to try to deal with the problem after it has arisen, by stocking ponds with predators. A range of predator species and stocking densities have been used (Table 10.17) with varying success. However, high yields are often depressed and there may also be difficulties in obtaining the appropriate predator species in adequate numbers.
Table 10.17
FISH PREDATORS USED FOR THE EFFECTIVE CONTROL OF
RECRUITMENT OF TILAPIAS
(Guerrero, 1982)
| Prey species (tilapias) | Predator species | Stocking Ratio (predator:prey) |
| O. mossambicus | Elops hawaiiensis | 1:10 and 1:20 |
| Megalops cyprinoides | 1:10 | |
| Micropterus salmoides | ||
| O. niloticus | Cichla ocellaris | 1:15 |
| Clarias gariepinus | 1:10 | |
| Lates niloticus | 1:20–1:84 | |
| O. shiranus | Clarias sp. | 1:10–1:20 |
| T. rendalli | ||
| O. aureus | Cichlasoma managuense | 1:4–1:8 |
| Not specified | Hemichromis fasciatus | 1:48 |
Predation
Ponds are particularly susceptible to losses of stock through predation. Fish (e.g., Nile perch, catfish, snakeheads), amphibians (frogs, toads), reptiles (e.g., monitor lizards, iguanas, turtles, crocodiles), birds (king-fishers, herons, cormorants, etc.) and mammals (mink, otters, etc.) can all cause excessive losses of stock. Available control methods for ponds are summarized in Table 10.18. Caged fish may be readily protected with antipredator nets and top nets, the latter also being effective at protecting stock held in tanks and raceways.
Table 10.18
ANTI-PREDATOR DEVICES FOR PONDS
| Predators | Protection/amelioration of problem | Comments |
| Fish | Screening of inlets; regular and complete draining of ponds | |
| Amphibians | Brick barrier, topped by angled sheet of corrugated, galvanized steel (height 45–60 cm) | Fry ponds are particularly particularly susceptible |
| Birds | Use lines stretched across pond | System is expensive, and impossible for large ponds Harvesting is difficult. Other methods, such as shooting, are not effective |
| Mammals | Traps |
Diseases and parasites
Fortunately, tilapias are extremely hardy and unless stressed by excessively high or low temperatures or salinities, or by being badly fed or handled, do not often suffer from diseases.
A list of the more common pathogenic organisms and diseases encountered is given in Table 10.19. Some ectoparasites may be controlled by chemical means. White spot in ponds can be treated using malachite green (three treatments, on three consecutive days, in which the concentration in the pond is raised to 0.15 ppm), whilst Trichodina and Dactylogyrus may be killed using formalin at 250 ppm or potassium permanganate at 5 ppm.
Table 10.19
COMMON DISEASES AND DISEASE AGENTS
| Pathogen | Genus/species | Comments |
| Viruses | Rarely cause problems | |
| Bacteria | Flexibacteria (slime bacteria) | Can cause skin lesions syndrome under high or low temperatures |
| Aeromonas spp. | Cause haemorrhagic septicaemias | |
| Edwardsiella | ||
| Mycobacterium | Tuberculosis | |
| Fungi | Saprolegnia | Superficial infections of body, often associated with handling |
| Branchiomyces | Invades gills. Associated with high levels of decaying organic matter | |
| Aspergillus Penicillium | Granulomatosis renal infections in intensive culture | |
| Protozoa | Ichthyophthirius | Causes white spot and is associated with low temperatures |
| Myxosporidia | Common but rarely pathogenic | |
| Trichodina, Chilodonella | Present on most fish in small numbers. Cause problems when fish is stressed | |
| Ichthyobodo | Highly pathogenic parasite of juveniles. Occurs in high density culture | |
| Metazoa
| Gyrodactylus | Outbreaks have been recorded in ponds and raceways |
| Clinostomum | Metacercariae cause distortion (bulging) of body in fry | |
| Euclinostomum | Spoils appearance and increases susceptibility to stress | |
| Haplorchis | Mortalities can occur with heavy infestation | |
| Diplostomum | These eye flukes can cause cataracts and blindness | |
| Contracaecum | Nematode which, although rarely causes pathological effects, may affect marketability | |
| Argulus, Lernaea, and Ergasilus | Ectoparasitic crustaceans, which although do not cause mortalities, may affect marketability |
In addition to the above list, tilapias may suffer from a range of nutritional disorders, associated with vitamin-deficiency (vitamins C and E) in diets or aflatoxins in feed ingredients.
References
Balarin, J.D. and R.D. Haller. 1982 The intensive culture of tilapias in tanks, race-ways and cages. pp. 265–356. In: J.F. Muir and R.J. Roberts (eds.). Recent Advances in Aquaculture. Vol. 1. London, Croom Helm Ltd.
Guerrero, R.D. 1982 Control of tilapia reproduction. In: R.S.V. Pullin and R.H. Lowe McConnell (eds.). The biology and culture of tilapias. ICLARM Conf. Proc., (7): 309–16
Jauncey, K. and B. Ross. 1982 A guide to tilapia feeds and feeding. Stirling, Institute of Aquaculture, 111 p.
Philippart, J.Cl. and J.Cl. Ruwet. 1982 Ecology and distribution of tilapias. In: R.S.V. Pullin and R.H. Lowe McConnell (eds.). The biology and culture of tilapias. ICLARM Conf. Proc., (7): 15–60