by
K.N. BEDIRIAN,
E.B. BURNSIDE,
H. KANAGAWA
and
J. WILTON
Since the first successful embryo transfer in rabbits reported by Heape in 1890 in Cambridge, England, scientists in many parts of the world have used this technique quite successfully in other laboratory animals. In domestic animals transfers were reported by Warwick and Berry (1949) with sheep and goats, by Kvasnickii (1951) with pigs, and by Willett et al. (1951) with cattle. During the 1950s and 1960s further successful transfers were reported in domestic animals. These encouraging reports, together with that of Rowson et al. (1969) from Cambridge, England, led to the commercialization of this practice in cattle. Because cows (like some other species of farm animals) can be made to superovulate by hormonal treatment. this technique can be used to produce an increased number of calves per cow in a given period. It should thus be possible to multiply the progeny of a desirable female individual in somewhat the same way as artificial insemination does for the male. Although the technique is applicable to a number of domestic animals, the present article deals primarily with cattle since. it is with this species that embryo transfer is now practised on a commercial scale. The major steps involved in an embryo transfer operation in cattle are as follows:
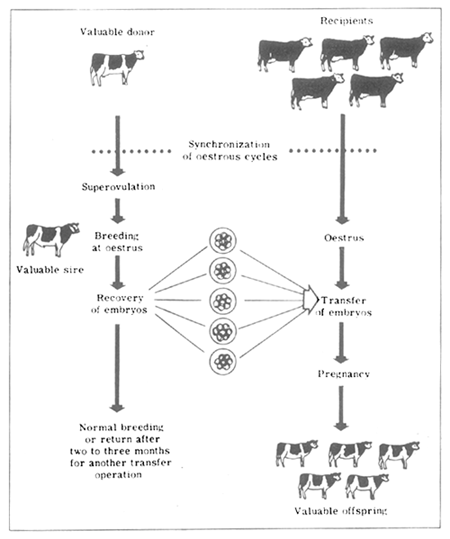
The donor cow is stimulated with a hormone (generally pregnant mare's serum gonadotrophin (PMSG) or follicle-stimulating hormone). Following such stimulation the animal comes on heat and she is bred or artificially inseminated with semen of the breeder's choice. In order to obtain maximum fertility two breedings are generally employed with a 12–hour interval, and a higher dose of semen is used the second time. It is expected that the donor will release a number of ova (10 to 15 or more) at this oestrus instead of the usual one. The ova are naturally fertilized within the donor and proceed to develop. If these embryos are left to grow in the donor cow, resorption and or multiple pregnancies may result which are undesirable in cattle because of resulting complications and the possible occurrence of freemartins. About four to five days after breeding (when the embryos are freely “floating” within the uterus and are not yet implanted), the embryos are flushed out with a suitable biological medium.
K.N. Bedirian and H. Kanagawa are with Modern Ova Trends Ltd., R.R.2, Norval, Ontario, Canada; J. Wilton and E.B. Burnside are at the Department of Animal and Poultry Sciences, University of Guelph, Ontario, Canada.
Embryo transfer is an artificial method of breeding whereby newly formed embryos prior to implantation are removed from a female animal and transferred into the reproductive tract of another female of the same species where they develop to term. The animal from which the embryos are removed is usually referred to as the donor, while the one that receives and carries the embryo is the recipient. The resulting offspring derive their genes from the donors from which they were removed and from the males to which the donors were bred.
Group of embryos at about the 32-cell stage shortly after recovery (enlargement approximately x 100).

This recovery procedure is best accomplished surgically, whereby the uterus of the donor animal is brought into view through an incision either through the midline or the flank. The operation takes about one and a half hours. Both the uterine horns are flushed by passing a tube through the uterine wall from one end and a hypodermic needle from the other; the medium is gently forced by a syringe and collected in a suitable dish. The embryos at this stage are still microscopic (approximately 0.2 mm in diameter) and are usually in the 32-cell stage of development. The dishes are then examined under a dissecting microscope. The embryos are picked up with a small amount of medium by a small glass pipette attached to a microsyringe and are then transferred surgically into the recipient animal's uterus by puncturing the uterine wall with a blunt needle and inserting the pipette carrying the embryo through it, followed by gentle pressure to eject the embryo into the uterine lumen. Embryos are transferred within four to five hours of recovery; during this time they are stored with minimum changes of temperature and pH.
It is important that the oestrous cycle of the recipient animal be synchronous with that of the donor (both donor and recipients should be in heat on the same day). In order to achieve this, it is necessary either to maintain a herd of approximately 250–300 regularly cycling animals to ensure about 12–15 recipients coming on heat every day to match with a given donor, or artificially synchronize the oestrous cycle of a smaller group of recipients to occur on a predetermined day when the donor is expected to come on heat. This can be achieved with a satisfactory rate of success by using various oestrus-synchronizing agents; prostaglandins have been found to be quite suitable and do not appear to impair fertility.
The basic steps involved in embryo transfer are diagrammatically represented in the graph on page 22.
Commercial feasibility
During the past few years a number of organizations have been set up in several countries, including Canada, the United States, the United Kingdom and Australia, to offer embryo transfer service to cattle breeders on a commercial basis. The skilled and specialized techniques necessary for a successful operation have set a relatively high cost for this service. Under current North American market conditions the value of offspring from cattle imported from continental Europe, such as Limousin, Simmental, Charolais, Blond d'Aquitain and Maine-Anjou, is high enough to justify the cost, which is about $2 000 for each operation plus $2 000 for every diagnosed pregnancy resulting from it. The value of a calf should thus be at least $4 000 to warrant the operation, although, of course, the greater the number of pregnancies obtained the lower the cost will be per calf.
According to current performance, it is possible to produce an average of five calves per donor cow in 15 months

1

2

3

4
1. Embryos are located and examined under the microscope prior to transfer. 2. Embryo recovery performed by a surgical team on a donor cow. 3. A recipient cow being prepared to receive an embryo. 4. Eight Charolais calves (5 females and 3 males) produced by the donor cow on the far right; some of the recipients that carried these calves can be glimpsed in the background.
At present the success rate obtained commercially averages between two and three pregnancies per operation. More than 65 calves were born and over 150 pregnancies have been established by the writer's services during the first year of their programme. There is great individual variability in response to treatments as well as in the number of established pregnancies. It has already been possible to produce more than a dozen pregnancies from one single collection of fertilized ova. The process of collection can be repeated quite successfully on the same cow. In the writers' programme at present two operations are usually performed on a donor within a two- to three-month period for the collection of fertilized ova, and they recommend that a natural pregnancy follow this. Based on their present success rates, it is therefore possible to produce an average of five calves per donor cow within a 15–month period.
The genetic implications of this for cattle breeding could be substantial. The time element involved in establishing a new herd or upgrading a cattle population under specified circumstances can be considerably reduced. The development of inbred female lines is possible for crossing purpose. Furthermore, one could use only the top 10 percent of the herd to provide replacement stock. It is important that only genetically superior females be propagated by this technique.
According to the current success rates the economic feasibility of embryo transfer in cattle is justified in certain circumstances. A number of technical problems do exist, however, and their solution may greatly advance knowledge and improve the efficiency of this technology, leading to its more widespread use. The following are the major problem areas in which further research is indicated.
Great individual variability exists among animals in characteristics related to successful ova transfers. In the writers' experience, approximately two thirds of the treated animals produce pregnancies. A number of factors influence the results, including response to superovulatory treatments, recovery rate of superovulated ova, the quality of resulting embryos and the methods employed in handling and transferring them. Oestrus synchronization in the recipient herd is very important in increasing the pregnancy rate of transferred embryos. These problems are currently under investigation by many researchers, and improvements are expected.
Postsurgical adhesions that may result in the donor animal should be considered, as they may impair her future reproductive ability. Fortunately, they can be minimized with practice. With the method of uterine flush employed in the writers' clinic only the uterine horns are handled during the flushing process, so that possible adhesions on the oviducts and fimbriae are minimized. Indeed, present results do not indicate any serious postoperative reproductive problems.
| Acknowledgements |
| The authors wish to acknowledge the valuable suggestions of Drs. D.C. Wilson and J.E. Robertson of Modern Ova Trends Ltd. |
However, it would be desirable to develop simpler techniques of recovery that present no hazards to the reproductive potential of the donor. Considerable research has been done in the past on nonsurgical techniques of recovery. Recent Japanese reports (Sugie, 1973) indicate some success, but the rates do not seem to be high.
Future of embryo transfer
The dimensions of development in the embryo transfer field are multiple and exciting. The commercial realization of the technique is very likely to have a greater impact on research. The present technology indicates a trend of improvement and refinement. One important area which is actively researched by many investigators, including the writers, is the storage of embryos by freezing, similar to sperm preservation. Very promising success rates have recently been obtained with frozen mouse embryos (Whittingham et al., 1972). Also, a live calf has been born from the transfer into a recipient cow, by researchers in Cambridge, England, of an embryo that had been frozen. The future in this area looks promising. Once bovine embryos are successfully frozen, banks can be established and the world can experience a new era of animal trade between countries. If embryos can be stored in a frozen state their immediate transfer becomes unnecessary. They can be transferred to the desired host animal anywhere and at any time. This could tremendously reduce present costs and make the practice accessible to many farmers.
In the meantime, it is possible that embryos can be shipped to other countries in a non frozen state, as it has been shown by the writers and others that they can survive under relatively simple laboratory conditions for as long as four days.
In vitro fertilization could be another area of development. The advantage here lies in the fact that the ovaries contain many more potentially viable eggs than can be utilized by the present system of in vivo fertilization. When abundant supplies of embryos become available from superior animals, even after slaughter, two could be transferred into one host cow, thus inducing artificial twinning which could have a great impact on meat production. Another possibility is the sexing of these embryos before transfer, so that the sex of the calf to be born can be predetermined. This has obvious implications in the dairy sector. It has also been shown that superovulation of immature females can be achieved; the resulting embryos when transferred to mature animals develop and grow into normal calves. A considerable shortening of the generation interval and the early progency testing of females are indicated here.
These and possibly other developments in embryo transfer are expected to play a significant role in world animal production in the coming years, with substantial benefits for both developed and developing countries.
REFERENCES
Heape, W. 1890. Preliminary note on transplantation and growth of mammalian ova within a uterine foster-mother. Proc. Roy. Soc., B48: 457.
Kvasnickii, A.V. 1951. Interbreed transplantation of ova. Anim. Breed. Abstr., 19: 224.
Rowson, L.E.A., Moor., R.M. & Lawson, R.A.S. 1969. Fertility following egg transfer in the cow: Effect of method, medium and synchronization of oestrus. J. Repord. Fert., 18: 517.
Sugie, T. 1973. Studies on techniques for artificial pregnancy in cattle. Japan, Dept. of Agriculture, Publication No. 62.
Warwick, B.L. & Berry, R.O. 1949. Intergeneric and intraspecific embryo transfers in sheep and goats. J. Hered., 40: 297.
Whittingham, D.G., Leibo, S.P. & Mazur, P. 1972. Survival of mouse embryos frozen to -196 and -296°C. Science, 178: 411.
Willett, E.L., Black, W.G., Casida, L.E., Stone, W.H. & Buckner, P.J. 1951. Successful transplantation of a fertilized bovine ovum. Science, 113: 247.