O.J. Torrissen
Matre Aquaculture Station, Institute of Marine Research, Matredal, Norway
ABSTRACT
A short review of hatching and rearing techniques of the salmon species, Salmo salar and Oncorhynchus spp., is given. Successful rearing depends on three main factors, water quality and supply, rearing facilities and satisfactory feed and feeding.
The salmonids demand pure water and particular attention has to be given to factors such as water temperature, dissolved oxygen and nitrogen, ammonia, the salt content and pollutants during the production.
The most widely used incubators and rearing ponds are described and given a short evaluation. Today the vertical flow incubator and hatchery troughs with trays are most popular and give the greatest flexibility. Rearing systems may be divided in three principal groups: man made ponds, pens in natural lakes and natural environment systems. Man made ponds are the dominating system, and during the last years circulating tanks have replaced the classical raceways in certain areas. Dry pelleted feed have nearly completely replaced the moist and semimoist feed for fry and fingerling production. The usual feed composition in commercial diets, the dietary requirements and feeding techniques are mentioned.
RESUME
En ce qui concerne les saumons, Salmo salar et Oncorhynchus. spp, sont résumés quelques facteurs importants et les méthodes d'incubation des oeufs et d'alevinage.
Sont indispensables: une quantité d'eau suffisante, pure et de bonne qualité aux points de vue température, taux d'oxygène et d'hydrogène, ammonium, sels minéraux, et le moins de pollution possible pendant la production.
Les différents incubateurs et les installations d'alevinage utilisées le plus fréquemment sont décrits et leur qualité est évaluée. L'incubateur en courant vertical et les bacs à panneaux perforés donnent les résultats les plus satisfaisants avec un maximum de flexibilité. Il existe trois systèmes d'alevinage: en étangs ou en bacs, en cages placées dans des eaux naturelles, et dans l'environnement naturel. Le premier système, dans lequel les bacs en courant circulant remplacent de plus en plus les bacs en courant longitudinal, est le plus généralement appliqué. Dans l'alevinage, les aliments secs ont presque complètement remplacé les aliments frais ou semi-humides. Leur composition normale ainsi que les besoins nutritifs des poissons sont indiqués.
Rearing of Atlantic salmon (Salmo salar) is carried out in northern Europe and on the east coast of the USA and Canada. Fry and fingerlings of the Pacific salmons, pink (Oncorhynchus gorbuscha), chum (O. keta), coho (O. kisutch), sockeye (O. nerka), and chinook (O. tshawytscha). are mainly reared in North America, Japan (mainly chum salmon) and the Soviet Union (mainly pink and chum salmon) (Table I).
Mass production is carried out for two purposes, i.e. the production for restocking of waters or for marine farming. Generally the same equipment and techniques can be used for both purposes, but usually the equipment is slightly modified according to the goal.
The fish must be genetically adapted to the environment: natural or artificial. Genetic improvement of salmon strains has been given great attention during the last years. Growth rate, age at sexual maturation, disease resistance, meat quality, egg size and probably age at smoltification depend on genetic factors (Møller and Nævdal, 1973; Refstie et al., 1977; Nævdal et al., 1978. In Norway selection for fast growth, late sexual maturity and early smoltification has been made, and may in future give a better suited Atlantic salmon for fish farming. In culture based fishery a high rate of recapture is essential, and factors increasing the rate of return are the basis for selection.
The growth of the anadromous salmon species is slower in fresh water than in seawater, and reducing the fresh water stage in production for seawater farms and for culture based fishery will increase the outcome. This may be done by intensive production, using high caloric feed and an optimal environment.
When releasing fish into rivers or sea, the physiological stage of the juvenile has to fit the environment. Factors such as stream flow, bottom substrate, predator pressure and adequate food supply have to be considered (Koski, 1972; Larsson, 1977 a; b; c; Prouzet, 1977; Prouzet et al., 1977). Generally the production and release therefore has to follow the natural cycle.
In this paper, methods for mass rearing of fry and fingerlings of salmonids are reviewed and different factors affecting the production are discussed based on the available literature.
The salmonids require pure water, and a physicochemical analysis of the water supply is indispensable before starting production, Particular attention has to be given to factors such as temperature, dissolved oxygen and nitrogen, salt content, ammonia, acidity and pollutants. A tolerance level is difficult to establish as it changes with fish size and species, and because of influences and interactions of other factors. Sublethal levels may also produce stress, and prolonged exposure may result in lowered resistance and greater susceptibility to diseases. Table II gives recommended levels for some of the water quality factors.
2.1.1 Temperature
Fish are poikilothermic and the metabolic rate is directly dependent on the water temperature. Within the optimum range maximum food conversion and growth occur (Burrows, 1972), and in intensive salmon farming temperatures near the optimum range are essential. During the start feeding, more fish are lost than in any other phase and, particularly at this time the temperature should be within the optimum range.
2.1.2 Oxygen
The oxygen consumption of salmonids is affected by temperature, fish size (Fig. 1) and physical as well as physiological activity.
An increase in water temperature results in an increase in the oxygen demand and at the same time the carrying capacity of the water is decreased (Burrows, 1972). It has been recommended that the oxygen level in the effluent water should not be lower than 5–6 mg/1 (Burrows, 1972; Westers and Pratt, 1977). However, it is not so much the absolute oxygen content, but the oxygen partial pressure (saturation level) which determines the diffusion through the gills and the saturation level of the hemoglobin (Fig. 2) and thereby the suitability of the water as a breathing medium (Rasmussen, 1968; Beamont, 1968). At high temperatures, therefore, the water demand is increased due to the decreased oxygen carrying capacity of both the water and the hemoglobin and the increased metabolic rate of the fish.
2.1.3 Nitrogen supersaturation
An oxygen level close to saturation is essential, but it is just as important to prevent gas embolism by avoiding supersaturation of nitrogen. Newly hatched fry are especially sensitive and the mortality can be very high even at a few per cent supersaturation (Peterson et al., 1972). Supersaturation is created when water is heated or when water and air are mixed under pressure. Deaeration of supersaturated water takes place when air and water are mixed at atmospheric pressure.
2.1.4 Salt tolerance
The Atlantic and Pacific salmons are anadromous. They hatch in fresh or brackfish water and migrate to sea at different stages depending on the species. Coho and pink salmon frequently spawn in the intertidal zone, and normal fertilization and development occur if the salinity does not exceed 18‰ (Holliday, 1969). During the yolk sac stage both species prefer fresh water, but soon after the resorption of the yolk sac they are able to tolerate seawater (Baggermann, 1960), and have therefore a great potential for rearing in areas with limited freshwater supply. The other salmon species seem to tolerate salinities at least up to the isotonic level (about 9‰) from the water hardening and onwards (Holliday, 1969; Saunders and Henderson, 1969; Szymelfenig, 1977), and full seawater after the parr-smolt transformation (Table III).
Adequate water supply is essential, and where possible the water should flow by gravity. Modern pumps are very reliable, but even a short power failure can cause a catastrophe and, if pumps are necessary, an independent standby system must be arranged.
2.2.1 Water sources
Surface water from rivers and lakes is usually saturated with oxygen, but the temperature changes with the air temperature. Higher winter temperatures are obtained by taking the water from deep in lakes, but the carbondioxide level may be high or the oxygen level low.
Infections by diseases (furunculosis, vibriosis etc.) and parasites (Costia, Myxosoma, Trichodina etc.) are a hazard when using water with a natural fish population or which is contaminated by water from other fish farms.
Wells and springs have nearly the same temperature throughout the year, and the gain in winter temperature may be lost in the summer. Such water is sometimes low in oxygen or it may be supersaturated with nitrogen.
2.2.2 Flow through systems
Such systems require large amounts of water and heating will be expensive if the heat is not conserved by heat exchangers or if cheap heat sources (thermal effluents from electric power plants or other industry) are not available. At the west and north coast of Norway the seawater temperature is relatively high during wintertime, and by running the freswater pipes through the sea an increase in temperature of about 3–6°C may be achieved.
2.2.3 Water reuse systems
Closed circuit systems solve problems with insufficient water supply and make controlling of the water environment factors economically favorable compared to flow through systems.
Reconditioning of the water (Table IV) includes reoxygenation, removal of solids and toxic waste products such as ammonia and carbondioxide. Sterilization to avoid infections and accumulation of toxic substances produced by microorganisms is also necessary.
During the embryological development the main purpose of water flow is to transfer oxygen to the eggs and to remove waste products. In environments where the bottom substrate gives shelter against the water currents, the alevins need smaller physical activity to keep their standing places than alevins in artifical environments without substrate (Brannon, 1965). Heavier fry and reduced mortality have resulted from use of matrix substrates during egg incubation and alevin development under hatchery conditions (Leon, 1975; Leon and Bonney, 1979). At any stage after hatching, the long term effect of too high water velocity is increased exercise and aggregation of the fish and thereby decreasing the growth rate.
Exposing eggs to light increases the mortality and gives smaller alevins at hatching (Brannon, 1965). Salmon eggs should therefore be incubated in darkness. Alevins show strongly photonegative responses and fry and fingerlings avoid bright daylight (Brannon, 1965). Protecting the fish against such light gives better distribution and thereby increased carrying capacity of the rearing tanks.
The photoperiod and light intensity are also shown to have influence on physiological processes such as growth rate (Knutsson and Grav, 1976 a; b), smoltification (Baggermann, 1960; Hazard and Eddy, 1950; Saunders and Henderson, 1970) and spawning.
The conventional hatchery incubators may be divided into two major categories:
Recently substrate incubator boxes (Fig. 3) have been developed particularly in British Columbia and Alaska (Bams and Simpson, 1977; Porter and Meerburg, 1977).
Type 1) are usually compact systems which require little space and water. Two principal incubators belonging to this system have practical use in large scale production, the cylinder incubator (Fig. 4) and the drip incubator. In the drip incubators the egg trays are alternated with perforated metal trays. Water introduced on the top drips over the eggs and provides enough moisture to maintain the embryos. Except for production of eyed eggs for sale, these incubator systems have small practical use.
The type 2) systems are more suitable and popular and comprise several types of incubators. Hatchery troughs (Fig. 5) where the eggs are spread on the bottom on a layer of limestone gravel are still used. Compared to other, modern systems available, much space and water are required. Egg baskets set in troughs (Fig. 6) in such a manner that the water is directed underneath the baskets and allowed to well up through the eggs to increase the egg carrying capacity. The rectangular meshes prevent the eggs from falling out, but allow the alevins to pass. After hatching the baskets are removed with all the dead eggs. In Norwegian and Swedish salmon farms trays rather than baskets are used. The trays with one of the sides and the bottom perforated (Fig. 7) hold back both eggs and alevins and thereby increase the carrying capacity of the troughs. This incubator usually consists of a 2.3 or 3.6 m long fibreglass trough containing 4 or 7 trays. Compared to other incubators the water requirement is relatively high.
In another modification, frames covered with a metal wire screen are stacked in deep troughs. Inspecting the eggs is difficult and the system has a limited use.
The vertical flow incubator (Fig. 8) has during the last 20 years been developed in USA. The most common design is made up of one or two superimposed shelves each one enclosing eight incubator trays. Each incubator tray is made up of an external basket in which there is an inner egg container tray. Troughs with trays (in northern Europe) and the vertical flow incubator (in America) are the most widely used incubators, and in table V these are compared to the cylinder incubator and a drip incubator with respect to carrying capacity and water and space requirement.
After the water hardening the eggs are resistant to slight mechanical disturbance for about 48 h, but after this period moving of the eggs should be avoided since the eggs then become progressively more vulnerable until the eyed stage. Open incubator systems such as the hatchery troughs, troughs with baskets and troughs with trays, have an advantage in allowing the water supply and eggs to be controlled during this period without disturbing the eggs. Dead and injuried eggs are an excellent medium for fungus. In the tender period removing of dead eggs is difficult, and malachite green (1:500000) is used to combat fungus. Especially in the cylinder incubator, fungus clods may cause damage by preventing the water flow in the relatively thick egglayer. Malachite treatment is therefore recommended every second or third day.
Eyed eggs are quite resistant to handling and during this stage a mechanical egg sorter (Winther & Borgbjerg, Hasselager, Denmark) may be used to remove the dead eggs.
Stress, both of eggs and alevins, increase the risk for diseases such as white spot, blue sac, malformations and fungus infections and should therefore be avoided (Brett, 1958; Egidius and Helland-Hansen, 1973).
Rearing systems for fry, fingerlings and smolt can be divided in three principal groups:
This system is the most widely used today. Relatively small ponds provide the greatest flexibility in management and tend to reduce the risk of catastrophic losses due to diseases, oxygen depletion and pollution.
Raceways, 25–35 m long, 3–10 m wide and 0.7–1 m deep appear to have a wide use in certain countries, especially in the USA. During the last years there has been a trend to replace the classical raceway by circulating tanks, particularly for startfeeding of Atlantic salmon, and in northern Europe the circulating tanks have completely replaced the raceways for all stages of salmon production. The advantages of the circulating tank are nearly uniform water circulation and consequently equal distribution of the fish, a better selfcleaning effect and a lower water requirement (Leitritz and Lewis, 1976). Circulating tanks may be cylindrical or rectangular with rounded corners (Fig. 9). Circular ponds usually have a diameter of 1–10 m and a depth of 0.4–1 m.
Modern ponds for salmon feeding are usually made up of inert materials as concrete, aluminium or fiberglass. It has never been possible to raise Atlantic salmon successfully in earthen ponds. Control and feeding is difficult to carry out and the lack of ability to clean them increases the risks for diseases (Peterson et al., 1972).
The design of this system has been adapted from systems used in the sea water rearing of salmon and trout. To secure water exchange the pens must be located in areas with natural current or else a mechanical substitute must be used. Even a thick ice layer does not harm the pens or the fish as long as it does not drift, and near 100% oxygen saturation has been reported in pens beneath 1 m ice (Møller, 1979).
Use of natural environment systems for the mass production of salmonid species has been developed. Such systems include rearing in artificial spawning channels, rivers and lakes.
Artificial spawning channels, mainly for Pacific salmon, have been constructed in North-America and similar channels may exist in Japan and the Soviet Union (Bardach et al., 1972). Adapting the fish to a natural environment and increasing the carrying capacity of rivers by preparing spawning and rearing areas is believed to offer greater advantages to the fishery than conventional hatchery and releasing methods (Volovik, 1966; Toivonen, 1977).
Rearing of Atlantic salmon smolt in lakes has succeeded (Harris, 1973) and has a great potential in producing fish for sea water farming and culture based fishery.
Systems for rational feeding must, however, be developed and for species with a long fresh water stage two or three localities of adequate size and design must be available to secure continuous production before rational smolt production can be achieved.
4.4.1 The stress factor
The effect of handling salmon (feeding, cleaning of tanks, etc.), noise, movements outside the tanks and fluctuations in light intensity are usually underestimated. The most striking effect of this type stress is growth retardation. In most cases fish in tanks are more exposed to stress of this type since cleaning of pens and natural environment systems is unnecessary, fish density is lower and the rearing area is usually greater.
4.4.2 Feed requirement
Møller (1975) showed that Atlantic salmon in rivers accept artificial food, that feeding increased the number of fishes at the feeding places and gave better growth rate. The expences of artificial feeds in a natural environment system can be reduced if the natural production i.e. the benthic production (Mundi, 1974; Williams et al., 1977) is increased.
4.4.3 Disease treatment
Treatments of diseases and parasites which can not be done orally is difficult to carry out in pens or natural environment systems. Fish in such systems must be transferred to enclosures where water exchange may be controlled. However, the lower density reduces the risk for catastrophic disease and parasite infections.
In commercial farming of fry, fingerlings and smolts dry pelleted feeds have almost completely replaced the wet and semimoist feeds. The dry pelleted diets consist of a mixture of fish meal, vegetable proteins, oils and a vitamin and mineral supplement.
Compared to wet and semimoist feeds, dry diets are desirable for many reasons: higher growth rate (Locke and Linscott, 1969; Bergstrøm, 1974; Ingebrigtsen, 1979), cheaper feeding, less contamination of ponds and water, less food waste, and practical automatic feeders are available.
5.1.1 Protein
The Salmonidae are carnivores and require a high level of high quality protein. The majority of experiments suggests that a level of crude protein of about 40% is satisfactory for feeding under a variety of conditions and for a number of species (Hastings and Dickie, 1972). The commercial dry diets usually have a protein content in the start feed of about 50%, which decreases to about 40% in feeds for bigger fish.
Fish meal constitutes the main protein source, (70–80%). Replacing fish meal (Herring meal) with cheaper protein sources, such as vegetable protein, have, so far, no success (Fowler and Banks, 1976 a and b). The most striking effect of low protein level or quality is growth retardation.
5.1.2 Carbohydrates
The digestibility of carbohydrates, especially undextrinated, is low in salmon species. Salmon can, however, utilize relatively high levels of carbohydrates without physiological upset, although liver glycogen increases with increasing carbohydrate levels in the feed. The long term effects of these liver changes have not yet been determined (Phillips, 1972; Pieper and Pfeffer, 1978). Commercial dry diets usually contain 10–20% carbohydrates in the start feed and somewhat more for bigger fish.
5.1.3 Fat
An average natural diet for salmon contains about 65% protein and 12% fat (between 5.5 and 22.2%). However, the different types of fat as well as the content used in artificial fish feeds have given varying results with respect to the growth and the well being of the fish (Bergstrøm, 1974). Many of these early salmon feeds contained vegetable oils or hard animal fat as only fat source. Fish require fat of the ω3 type (Lee and Sinnhuber, 1972; Cowey and Sargent, 1972 and 1977), and maximum growth rate without deficiency symptoms can be obtained with ω3 alone, but not with ω6 alone. At a practical level it is still not known with certainty how much ω6 acids which can be safely incorporated into diets without upsetting the metabolism of the ω3 acids. This is important in the sense that most of the readily available vegetable oils have high levels of ω6 acids and marine oils are high in ω3 (Cowey and Sargent, 1972). Bergstrøm (1974) reports that Atlantic salmon fed with a commercial dry diet containing 16% fat (marine and lechitine) showed excellent growth and survival compared to fish given a diet containing 8% fat. Commercially available feed for Atlantic salmon fry today usually contains 16–20% fat, mainly of marine origin.
Unsaturated fats are vey liable to oxydation. The oxydation, products may be toxic (cause lipoid liver degeneration) or reduce the available level of other nutrients because of the reactivity of the fatty acid peroxides. Fish feed should therefore be protected against oxydation (Castell, 1978).
5.1.4 Minerals and vitamins
Very little is known about the mineral requirements of fish, and until proved otherwise all minerals essential to higher animals should be considered essential to fishes.
However, the vitamin requirements of salmon are well known, mainly thanks to the work of Halver (1957). He determined the requirement of 10 vitamins for chinook salmon. The requirements for Atlantic, chinook and coho salmon are given in table VI.
Growth rate is closely related to food particle size (Ingebrigtsen, 1977). Thorpe and Wankowski (1978) determined that the optimum particle size for Atlantic salmon was “0.025 × fish fork length”. Commercial dry pelleted feeds have a wide size distribution and will satisfy fish of a wide size range. It is, however, necessary to grade the fish in order to attain maximum growth by preventing competition for feed between fish of different sizes and to avoid cannibalism.
In order to achieve maximum utilization, the amount of feed required is related to water temperature, fish size, species and diet quality. The best feed utilization is, however, achived by feeding slightly less than maximum consumption (Fig. 10). For maximum growth, the fish should be fed slightly more than maximum consumption.
The first 2–3 months after yolk sac resorption is the most critical for the survival of salmon. In this period the mobility is low and if the feed is not evenly distributed and present in sufficient amounts the damage is irreparable. Feeding frequently is the best way to ensure that food is available. When hand feeding is practised, small salmon are usually fed 6–12 times every day, and at least 3–4 times a day for bigger fish. With automatic feeding, the frequency is made higher, depending on the feeder used.
ACKNOWLEDGEMENT
I am indebeted to, Dr. M. Holm, Dr. O. Ingebrigtsen, Dr. D. Møller and Ir. G. Nævdal of the Institute of Marine Research, Bergen, for critical reading of the manuscript.
Baggermann, B., 1960 Salinity preference, thyroid activity and the seaward migration of four species of Pacific salmon. J. Fish. Res. Bd. Can., 17 (3): 295 – 322.
Bams, R.A. and K.S. Simpson, 1977 Substrate incubators workshop-1976. Report on current state-of-the-art. Fish. Mar. Serv. Tech. Rep., 689: 67 p.
Bardach, J.E., J.H. Ruyter and W.O. Mclarney, 1972 Aquaculture. New York, Wiley-Interscience. 868 pp.
Beaumont, C., 1968 Unpublished data cited in: D.J. Randall, Gass exchange in fish. In: W.S. Hoar and D.J. Randall, Fish physiology. New York, Academic Press. 1970 (IV): 253 – 292.
Bergstrøm, E., 1974 The role of nutrition in growth and survival of young hatchery reared Atlantic salmon. Swedish Salmon Res. Inst. Rep., 4 (1): 265 – 282.
Brannon, E.L., 1965 The influence of physical factors on the development and weight of sockeye salmon embryos and alevins. Int. Pacific Salmon Fish. Comm. Prog. Rep., (12): 26 pp.
Brett, J.R., 1952 Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J. Fish. Res. Bd. Can., 9 (6): 265 – 323.
Brett, J.R., 1958 Implications and assessments of environmental stress. In: P.A. Larkin, The investigations of fish-power problems. Vancouver, H.R. McMillan Lectures in Fisheries, Univ. of British Columbia, 69 – 83.
Brett, J.R., J.E. Shelbourn and C.T. Shoop, 1969 Growth rate and body composition of fingerling sockeye salmon, Oncorhynchus nerka, in relation to temperature and ration size. J. Fish. Res. Bd. Can., 26 (9): 2363 – 2394.
Buckley, J.A., 1978 Acute toxicity of un-ionized ammonia to fingerling coho salmon. Prog. Fish-Cult., 40 (1): 30 – 32.
Burrows, R.E. and H.H. Chenoweth, 1970 The rectangular circulating rearing pond. Prog. Fish-Cult., 32 (2): 67 –80.
Burrows, R.E., 1972 Salmonid husbandry techniques. In: J.E. Halver, Fish nutrition. New York, Academic Press, 375 – 402.
Castell, J.D., 1979 Review of lipid requirements of finfish. In: J.E. Halver and K. Tiews (Eds.): Finfish Nutrition and Fishfeed Technology I: 59 –84. Heenemann GmbH & Co, Berlin 42.
Combs, B.D., 1965 Effect of temperature on the development of salmon eggs. Prog. Fish-Cult., 27 (3): 134 – 137.
Conrad, J.F., R.A. Holt and T.D. Krebs, 1975 Ozon disinfection of flowing water. Prog. Fish-Cult., 37 (3): 134 – 135.
Conte, F.P., 1969 Salt decretion. In: W.S. Hoar and D.J. Randall, Fish physiology. New York, Academic Press, (I): 241 – 292.
Cowey, C.B. and J.R. Sargent, 1972 Fish nutrition. Adv. mar. Biol., 10: 383 – 492.
Cowey, C.B. and J.R. Sargent, 1977 Lipid nutrition in fish. Comp. Biochem. Physiol., 57B: 269 – 273.
Dudoroff, P. and M. Katz, 1953 Critical review of literature on the toxicity of industrial wastes and their components to fish. Sewage and Industrial Wastes, 25 (7): 802 – 839.
Egidius, E. and O. Helland-Hansen, 1973 Produksjon av egg of yngel. Fisken of Havet, ser. B. (11): 1 – 129.
Elson, P.F. et al., 1973 Impact of chemical pollution on Atlantic salmon in North-America. International Atlantic Salmon Foundation Spes. Publ. Ser. 4 (1): 83 –110.
Fowler, L.G. and J.L. Banks, 1976a Animal and vegetable substitutes for fish meal in the Abernathy diet. Prog. Fish-Cult., 38 (3): 123 – 126.
Fowler, L.G. and J.L. Banks, 1976b Fish meal and wheat germ meal substitutes in the Abernathy diet. Prog. Fish-Cult., 38 (3): 127 – 130.
Halver, J.E., 1957 Nutrition of salmonoid fishes III, Water soluble vitamin requirements of chinook salmon. J. Nutr., 63: 225 – 243.
Halver, J.E., 1978 Vitamin requirements of finfish. In: J.E. Halver and K. Tiews (Eds.): Finfish Nutrition and Fishfeed Technology I: 45 – 58. Heenemann GmbH & Co., Berlin 42.
Harris, G.S., 1973 Rearing smolts in mountain lakes to supplement salmon stocks. Int. Atlantic Salmon Foundation Spes. Publ. Ser., 4 (1): 237 – 252.
Hastings, W.H. and L.M. Dickie, 1972 Feed formulation and evaluation. In: J.E. Halver, Fish nutrition. New York, Academic Press, 327 – 374.
Hazard, I.P. and R.E. Eddy, 1956 Modification of the sexual cycle in brook trout (Salvelinus fontinalis) by control of light. Trans. Am. Fish. Soc., 80: 158 – 162.
Holliday, F.G.T., 1969 The effects of salinity on the eggs and larvae of teleosts. In: W.S. Hoar and D.J. Randall, Fish physiology. New York. Academic Press, (I): 293 – 311.
Ingebrigtsen, O., 1977 Particle size. Internal report, Matre Aquaculture Station, Institute of Marine Research, Matredal-Norway.
Ingebrigtsen, O., 1979 Startforing av sjøaure (Salmo trutta) på tre typer fór ved 6 og 10°C vanntemperatur. To be published in Fisken og Havet ser. B.
Knutsson, S. and T. Grav, 1976 a Seawater adaption in Atlantic salmon (Salmo salar L.) at different experimental temperatures and photoperiods. Aquaculture 8: 169 – 187.
Knutsson, S. and T. Grav, 1976 b Growth and seawater adaptation in Atlantic salmon raised at different experimental photoperiods. Ices, C.M. 1976/E39: 10 p.
Kosko, K.V., 1972 A summary of the spawning channel research by F.R.I. as related to the effects of gravel composition and spawner density success, amergence survival and fry quality. Alaska Dep. Fish. Game. Inf. Leafl. 161: 84 – 90.
Larsson, H.O., 1977 a The influence of predation after release on the results of salmon smolt planting. Ices, C.M. 1977/M44: 9 p.
Larsson, P.O., 1977 b Size dependent mortality in salmon smolt plantings. Ices, C.M. 1977/M43: 5 p.
Larsson, P.O., 1977 c The importance of time and place of release of salmon and sea trout on the results of stockings. Ices, C.M. 1977/M42: 4 p.
Lee, D.J. and R.O. Sinnhuber, 1972 Lipid requirements. In: J.E. Halver, Fish nutrition. New York. Academic Press: 145–180.
Leitritz, E. and R.C. Lewis, 1976 Trout and salmon culture. Calif. Dept. Fish and Game, Fish Bull., 264: 197 p.
Leon, K.A., 1975 Improved growth and survival of juvenile Atlantic salmon hatched in drums packed with a labyrinthine plastic substrate. Prog. Fish-Cult., 37(3): 158–163.
Leon, K.A. and W.A. Bonney, 1979 Atlantic salmon embryos and fry: Effects of various incubation and rearing methods on hatchery survival and growth. Prog. Fish-Cult., 41(1): 20–25.
Liao, P.B. and R.D. Mayo, 1972 Salmonid hatchery water reuse systems. Aquaculture, 1: 317–335.
Lindroth, A., 1942 Undersøkingar øver befruktnings och utvecklingsførhållanden hos lax (Salmo salar). Meddelan från Statens Undersøkings- och Forsøksanstalt før Søtvattenfisket, 19: 1–29.
Locke, D.O. and S.P. Linscott, 1969 A new diet for landlocked Atlantic salmon and lake trout. Prog. Fish-Cult., 31(1): 3–10.
McNeil, W.J., 1976 Review of transplantation and artificial recruitment of anadromous species. FAO Tech. Conf. Aquacult., 76:R24: 13 p.
Mundie, J.H., 1974 Optimization of the salmonid nursery stream. J. Fish. Res. Bd. Can., 31(11): 1827 – 1837.
Møller, D., 1957 Kunstig fóring av yngel of ungfisk av laks og aure i fri elv. Dr. thesis, University of Oslo.
Møller, D., 1979 Personal communication. Senior scientists in aquaculture. Institute of Marine Research, Bergen-Norway.
Møller, D. and G. Nævdal, 1973 Variasjoner i yngelvekst hos laks of regnbueaure. Fisken of Havet ser. B (3): 21 p.
Nævdal, G. et al., 1978 Growth rate and age at sexual maturity of Atlantic salmon smoltifying at one or two years of age. Ices, C.M. 1978/F23: 8 p.
Peterson, H.H., O.T. Carlson and S. Jonasson, 1972 The rearing of Atlantic salmon. Astra-Ewos AB, Sødertelje, Sweden: 39 p.
Phillips, A.M. Jr., 1972 Calorie and energy requirement. In: J.E. Halver, Fish nutrition. New York. Academic Press : 2 – 28.
Pieper, A. and E. Pfeffer, 1978 Carbohydrates as possible sources of dietary energy for rainbow trout. In: J.E. Halver and K. Tiews (Eds.): Finfish Nutrition and Fishfeed Technology I: 209 – 219. Heenemann GmbH & Co, Berlin 42.
Porter, T.R. and D.J. Meerburg, 1977 Upwelling incubation boxes for Atlantic salmon. Ices, C.M. 1977/M: 22 p.
Prouzet, P., 1978 Relationship between density and growth of Atlantic salmon reared in nursery streams in natural conditions. Ices, C.M. 1978/M13: 13 p.
Prouzet, P. et al, 1977 Extensive production of Atlantic salmon parrs (Salmon salar L.) in four nursery streams of Northern Brittany. Ices, C.M. 1977/M18: 25 p.
Rasmussen, C.J., 1967 Håndbog i ørredopdr aet. Copenhagen, Rhodos: 241 p.
Refstie, T., T.A. Steine and T. Gjedrem, 1977 Selection experiments with salmon, II, Proportion of Atlantic salmon smoltifying at 1 year of age. Aquaculture, 10: 231 – 242.
Risa, S. and H. Skjervold, 1975 Water re-use system for smolt production. Aquaculture, 6: 191 – 195.
Sato, R., 1972 Saltwater and freshwater pond rearing of chum salmon. Alaska Dep. Fish and Game Inf. Leaf., 161: 101 – 102.
Saunders, R.L. and E.B. Henderson, 1969 Survival and growth of Atlantic salmon fry in relation to salinity and diet. Fish. Res. Bd. Can. Techn. Rep. 148: 7 p.
Saunders, R.L. and E.B. Henderson, 1970 Influence of photoperiod on smolt development and growth of Atlantic salmon (Salmo salar). J. Fish. Res. Bd. Can. 27: 1295 – 1311.
Szymelfenig, M., 1977 Influence of different sea water salinities on rainbow trout (Salmo gairdneri Rich.) and sea trout (Salmo trutta trutta L.) spermatozoons and eggs. Ices, C.M. 1977/M11: 7 p.
Thorpe, J.E. and J.W.J. Wankowski, 1978 Feed presentation and food particle size for juvenile Atlantic salmon, Salmo salar L. In: J.E. Halver and K. Tiews (Eds.): Finfish Nutrition and Fishfeed Technology I: 501 – 514. Heenemann GmbH & Co, Berlin 42.
Toivonen, J., 1977 Differences in recapture of wild hatchery reared salmon smolt. Ices, C.M. 1977/M7: 5 p.
Volovik, S.P., 1966 Concerning the release of young pink and chum salmon from Fish Hatcheries. Fish. Res. Bd. Can. Transl. Ser., 713: 5 p.
Westers, H. and K.M. Pratt, 1977 Rational design of hatcheries for intensive salmonid culture, based on metabolic characteristics. Prog. Fish-Cult., 29(4): 157 – 165.
Williams, D.D., J.H. Mundie and D.E. Mounce, 1977 Some aspects of bentic production in a salmonid rearing channel. J. Fish. Res. Bd. Can. 34: 2133 – 2144.
Table I. Approximate numbers of juvenile salmon released annually into marine basins
| Millions of juveniles | |||||||
| Atlantic salmon | Pink | Chum | Coho | Sockeye | Chinook | References | |
| USA | 1 | 3 | 10 | 78 | 2 | 212 | Mc Neil (1976) |
| Canada | 1 | 20 | 55 | 1 | 90 | 1 | " |
| Soviet Union | 2 | 404 | 201 | " | |||
| Japan | 39 | 785 | 1 | 1 | 1 | " | |
| Sweeden and Finland | 2 | " | |||||
| Republic of Korea | 1 | 2 | " | ||||
| Approximate total | 9 | 466 | 1052 | 82 | 93 | 214 | |
Some of these statistics date back several years, so the estimates are probably conservative indicators of current levels of hatchery production. They indicate, nevertheless, relative levels of artificial recruitment for the various species and geographical areas.
Table II. Tolerance and optimum levels for some environmental factors
| Minimum level | Maximum level | Optimum level | References | ||
| TEMPERATURE | |||||
Atlantic salmon | |||||
eggs | 0.5°C | 10°C | 6–8°C | Egidius and Helland-Hansen(1973) | |
Startfeeding | 7°C | Peterson et al. (1972) | |||
fry, fingerlings | -0.5°C | 24°C | 10–18°C | Liao and Mayo (1972) | |
Pacific salmon | |||||
eggs (Chinook, sockeye) | 4.5–6°C | 13–14°C | Combs (1965) | ||
fry, fingerlings | >0°C | 24–25°C | 12–14°C | Brett (1952) | |
| OXYGEN SATURATION | 65%1 | 90–100%2 | 1) Peterson et al. (1972) 2) Liao and Mayo (1972) | ||
| NITROGEN SATURATION | 100–105% | Liao and Mayo (1972) | |||
| AMMONIA - unionized | 201-12.52 | μg/1 | 1) Buckley (1978) 2) Westers and Pratt (1977) | ||
| NITRITE | 200 | " | Liao and Mayo (1972) | ||
| CHLORINE | 200–500 | " | Egidius and Helland-Hansen (1973) | ||
| COPPER* | 48 | " | < 1 μg/1 | Elson et al. (1972) | |
| ZINK* | 600 | " | <12 " | ||
| LEAD | 100–500 | " | Doudoroff and Katz (1953) | ||
| pH | 5.4 | 6–8 | Egidius and Helland-Hansen (1973) |
* in combination the effect is addidative
Influences and interactions of other environmental factors and variation in tolerance and optimum levels between the different species and strains makes it difficult to set absolute values.
Table III. Comparative survival for certain species of salmonids at different salinities for various sizes.
| Median survival time of exposed population (hr) | ||||||||||
| Stage in life history | size range (cm) | 30‰ | 28‰ | 26‰ | 24‰ | 22‰ | 20‰ | (0–5‰) | References | |
| Atlantic salmon | Fry | 3–4 | 4 | 7 | 10 | 22 | 50 | - | ∞ | Conte (1969) |
| " " | Parr | 7–10 | 19 | 30 | 72 | - | - | - | ∞ | " |
| " " | Smolt | 12–15 | 100 | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | " |
| Sockeye | Fry | 3–4 | 72 | 150 | 720 | 720 | 720 | 720 | ∞ | " |
| " | Parr | 5–6 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| " | Smolt | 6–8 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| Chinook | Fry | 3–4 | 60 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| " | Parr | 5–6 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| " | Smolt | 7–15 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| Coho | Fry | 3–4 | 44 | 48 | 720 | 720 | 720 | 720 | ∞ | |
| " | Parr | 7–8 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| " | Smolt | 9–15 | 720 | 720 | 720 | 720 | 720 | 720 | ∞ | |
| Chum | Fry | 3–4 | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | Limited | Holliday (1969) |
| Pink | Fry | 3–4 | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | " * | Sato (1972) *Baggermann (1960) |
Table IV. Treatment for recondition of water
| Treatment | Process | References | |
| REMOVING OF WASTE PRODUCTS: | |||
SOLIDS | sedimentation/filtration | Liao and Mayo (1972) | |
AMMONIA-UNIONIZED | pH - control |  | Westers and Pratt (1977) |
AMMONIA | Biological filtration1 | 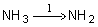 | Leitritz and Lewis (1976) |
NUTRITE | Biological filtration2 | 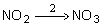 | " " " |
CARBONDIOXIDE | Aeration | washing out | Liao and Mayo (1972) |
MICROORGANISMS | uv-light | sterilization | Risa and Skjervold (1975) |
| Ozone | sterilization | Conrad et al. (1975) | |
| Chlorine3 | sterilization | ||
| OXYGENATION | Aeration | reoxygenation | Liao and Mayo (1972) |
1) Nitrosococcus and Nitrosomonas
Table V. Comparison of four hatchery systems
| System | Producer | Space-requirement | Amount of eggs | Water-requirement | ||||
| Length cm | width cm | area m2 | 1/unit | 1/m2 | 1/unit | 1/1 eggs | ||
| Hatcherytroughs | EWOS | 220 | 50 | 1.1 | 3.0 | 2.7 | 3 | 1 |
| EWOS | 360 | 50 | 1.8 | 5.5 | 3.0 | 5 | 0.9 | |
| Troughs with trays | EWOS | 220 | 50 | 1.1 | 6.0 | 5.5 | 8 | 1.3 |
| EWOS | 360 | 50 | 1.8 | 10.5 | 5.8 | 12 | 1.1 | |
| Vertical flow | HEATH TECHNA | 60 | 60 | 0.4 | 12 | 30 | 10–35 | 0.8–2.9 |
| Drip incubator | EUVANG | 50 | 70 | 0.4 | 15 | 32.5 | 2 | 0.13 |
| Cylinder incubator | BAKKELITTFABR. | - | 50 | 0.3 | 30 | 100 | 10 | 0.3 |
Modified from: Egidius and Helland-Hansen (1973)
Table VI. Vitamin requirements for growtha (from Halver, 1978)
| Vitamin (mg/kg dry diet) | Atlantic salmon | Chinook salmon | Coho salmon |
| Thiamin | 10–15 | 10–15 | 10–15 |
| Riboflavin | 5–10 | 20–25 | 20–25 |
| Pyridoxine | 10–15 | 15–20 | 15–20 |
| Pantotenate | Rb | 40–50 | 40–50 |
| Niacin | R | 150–200 | 150–200 |
| Folacin | 5–10 | 6–10 | 6–10 |
| Cyanocobalamin | R | 0.015–0.02 | 0.015–0.02 |
| myo-Inositol | R | 300–400 | 300–400 |
| Choline | R | 600–800 | 600–800 |
| Biotin | 1–1.5 | 1–1.5 | |
| Ascorbate | R | 100–150 | 50–80 |
| Vitamin A | R | R | |
| Vitamin Ec | 40–50 | R | |
| Vitamin K | R | R |
a Fish fed at reference temperature with diets at about protein requirement.
c Requirement directly affected by amount and type of unsaturated fat fed.
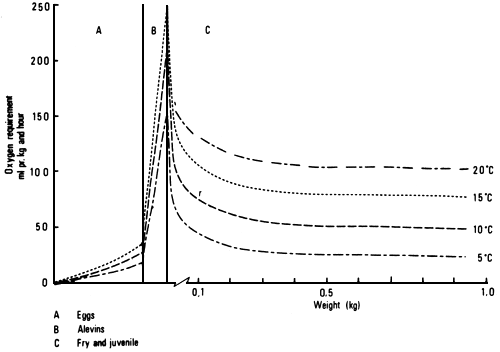
Fig. 1. Oxygen demand of Atlantic salmon in relation to temperature. After Lindroth (1942).
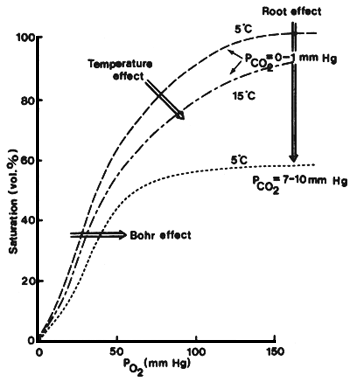
Fig. 2. Oxygen dissociation curves for the trout, Salmo gairdneri. Modified from: Beaumont (1968).
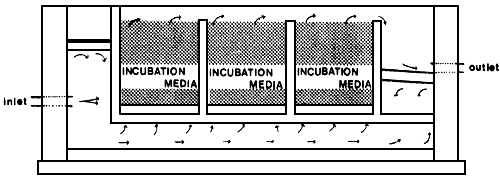
Fig. 3. Upwelling incubation box (“Exploits River Box”). The outside dimensions are 4.79 × 2.07 × 1.92 m deep and the three incubation compartments 0.91 × 1.52 × 1.37 m deep. Twelve to eighteen layers of 10000 to 12000 eggs are deposited between layers of gravel or turf (152000–304000 eggs/m3 substrate). Modified from: Porter and Meerburg (1977).
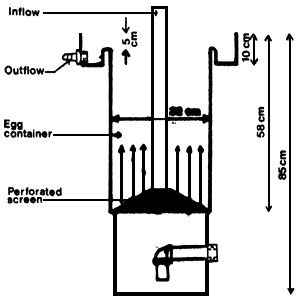
Fig. 4. Hatchery cylinder. The arrows indicate the water flow.

Fig. 5. Hatchery trough. The arrows indicate the direction of water flow. The eggs are deposited on a layer of limestone gravel.
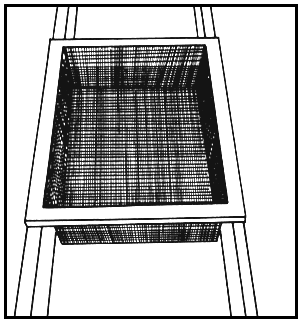
Fig. 6. Eggbasket in trough. Modified from: Leitritz and Lewis (1976).
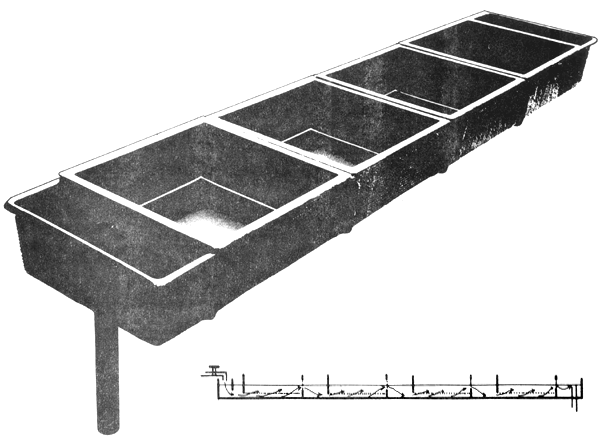
Fig. 7. Hatchery trough with trays. The arrows indicate the water flow.
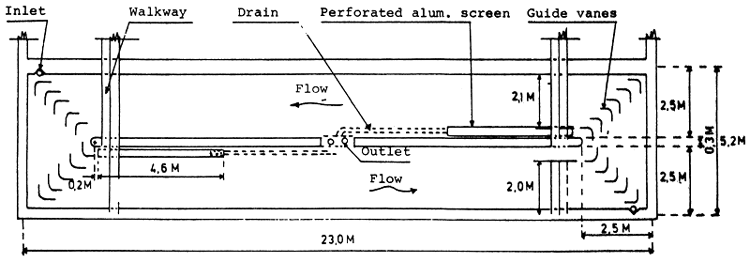
Fig. 9. Rectangular circulation pond. Modified from: Burrows and Chenoweth (1970).
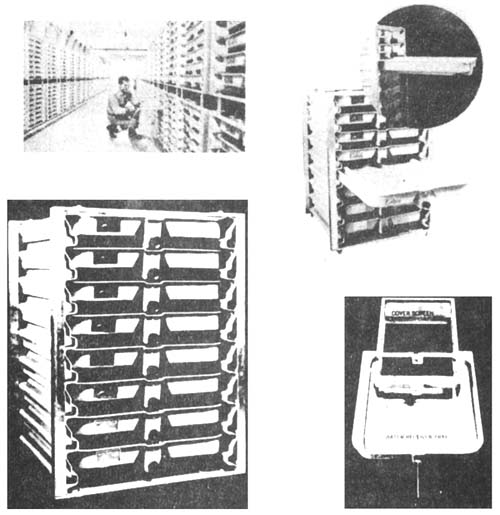
Fig. 8. The vertical flow incubator. (Heath Techna).
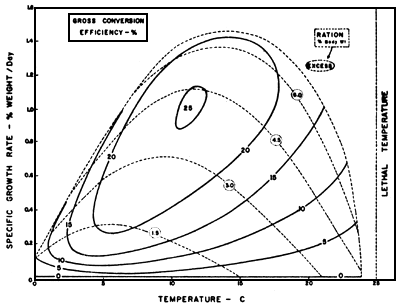
Fig. 10. Gross efficiency of food convertion in relation to temperature and ration for sockeye salmon. (Brett 1969).