M.C.M. Beveridge - Institute of Aquaculture, University of Stirling (Scotland)
This report forms part of the remit of the EIFAC Working Party on the Prevention and Control of Bird Predation in Aquaculture and Fisheries Operations. Its primary purpose is to review the literature on bird predation, although it also considers the broader aspects of bird/aquatic ecosystem interactions, particularly as they affect fish populations (e.g., disease transmission, trophic state, etc.). It is thus intended to complement the summary reports on the status of the problems experienced by EIFAC member states prepared from questionnaires.
The report sets out to determine the avian species involved, and to assess the economic losses incurred in open water fisheries, pond fish farms and trout farms. It also examines the effectiveness of various methods of control. The report draws heavily upon published data from within EIFAC member states, but also includes information from outwith this area, particularly on control methods.
An examination of handbooks and field keys to European birds (e.g., Cramp et al., 1977, 1980, 1983, 1985) reveals more than 100 species, including divers, grebes, herons, cormorants, wildfowl, hawks, eagles, cranes, gulls, swifts, kingfishers and swallows which are associated with wetlands, using them as a source of food and/or shelter. Many of these species, however, are rare (e.g., Gavia adamsii, the white billed diver), whilst others, such as the white pelican Pelecanus onocrotalus are migrants, occurring in only a few of the EIFAC countries for limited periods of time each year. Yet others, such as the swift (Apus apus), have only minimal contact with water whilst in flight and tend to associate with water bodies only under certain circumstances, such as during a hatch of aquatic insects (Lack and Owen, 1955). These species are unlikely to have any serious adverse effect on fish stocks or fisheries.
A number of species, however, may be regarded as common, are widely distributed and have a strong association with wetlands, using them for both nesting and feeding purposes. Several other species, such as the crow, Corvus corone cornix, which exhibit opportunistic or scavening feeding behaviour, are attracted to fish farms (Keve, 1962) where dead fish and fish food may be readily available.
The impact of birds on the aquatic environment may be summarized as shown in Figure 1. Some of these impacts are minimal and are unlikely to have any significant effect on fish populations (e.g., habitat modification through nest building). However, other activities, such as feeding or roosting, may indirectly affect fish through their role in disease transmission and their impact on trophic state, whilst piscivorous feeding directly influences fish numbers and may even affect community structure.

Figure 1 The effects of birds on fish
In this report, the impact of birds on trophic state, disease transmission and predation will be examined, and it concludes with a review of available methods for limiting adverse effects on freshwater fish communities.
Predation by birds may affect fish populations indirectly through competition for food, or directly through piscivory.
3.1 Plants
Most species of waterfowl rely predominantly on aquatic plants, belonging to the genera Chara, Potamogeton, Ceratophyllum, Lemna, Typha, Elodea and Phragmites for food (Gaevskaya, 1969). However, although they can under exceptional circumstances consume up to 90% of the annual macrophyte production (Uspienski, 1967, in Dobrolowski, 1973), they usually account for only 2–3% of production (Dobrolowski, 1973; Dobrolowski, Halba and Nowicki, 1976). Consumption of aquatic plants on this scale by birds is unlikely to have any significant adverse effect on fish populations which generally use fringing macrophyte beds for breeding, nursery areas, shelter or foraging rather than as a direct source of food.
3.2 Invertebrates
Invertebrates (principally insects) play a dominant role in the diet of juvenile and adult dabbling ducks (Tribe: Anatini) during the summer months (Danell and Sjoberg, 1980) and are also an important component in the diets of divers, herons, smew, coots, gulls and kingfishers at certain times of the year or during certain periods of their life (see below). According to Dobrolowski, Halba and Nowicki (1976) invertebrates account for between 10% and 36% of the diet of wildfowl communities in Polish lakes, the birds consuming between 15 and 60 kg/ha of invertebrate production each year. The relative importance of invertebrates in the diet was found to increase with lake productivity.
Studies in Sweden by Eriksson (1979) and in Canada by Eadie and Keast (1982) have shown that goldeneye (Bucephala clangula) and perch (Perca fluviatilis) have a high diet overlap in terms of invertebrate prey type and size, and that there was a reciprocal density trend which could not be solely attributed to differences in habitat use between the two species. Competition for food between black duck (Anas rubripes) and brook trout (Salvelinus fontinalis) has also been demonstrated in the USA (Hunter et al., 1986).
In view of the quantities of invertebrates that birds consume, it is likely that they do compete with fish for prey, although the importance of competition and its effects remain largely unstudied.
3.3 Fish
The principal species of piscivorous birds associated with inland waters are summarized in Table 8. Amongst the divers (Family: Gaviidae), the red-throated (Gavia steluata) and black-throated (G. arctica) are commonest, and occur in remote inland waters during April–September/October, usually in small numbers. Both species feed principally on fish, including salmonids, roach (Rutilus rutilus), bleak (Alburnus alburnus), dace (Leuciscus leuciscus), perch and carp (Cyprinus carpio) (Madsen, 1957).
All grebes (Family: Podicepedidae) feed on fish, although the great-crested grebe (Podiceps cristatus) is the commonest and most widely dispersed of the European species and has a more piscivorous diet than other members of the family. It tends to inhabit large lakes during March–October, except in central European countries such as Switzerland where it overwinters in ice-free areas, and feeds on a wide range of small (3–21 cm; mean size= 13 cm) fishes (Geiger, 1957). Usually it prefers more than 1 ha per pair of birds, although occasionally several thousand pairs have been recorded at open water sites (Jacoby, Knotzsch and Schuster, 1970; Vlug, 1974; Geroudet, 1974).
There are several races of cormorant (Phalacrocorax carbo), including North Atlantic (Phalacrocorax carbo carbo), Eurasian (Phalacrocorax carbo sinensis), Moroccan (Phalacrocorax carbo maroccanus) and African (Phalacrocorax carbo lucidus) races, all of which are almost exclusively piscivorous in feeding habit. Phalacrocorax carbo carbo is non-migratory and has a large coastal distribution in western Europe, although it can occur up to 60 km inland (Mills, 1965). Phalacrocorax carbo sinensi, on the other hand, is a continental, largely migratory race, which now occurs widely throughout Europe, from east to west, and from north to south (Hansen, 1984). In inland areas both races are found on or around lakes, reservoirs, fish ponds, open water areas in swamps and broad sluggish areas of rivers, where they feed on a wide range of fish species (Madsen and Sparck, 1950; van Dobben, 1952; Mills, 1965; West, Cabot and Greer-Walkob, 1975; McIntosh, 1978; Ranson, 1982; Moerbeek et al., 1987). Typically, the cormorant is a solitary feeder, although it sometimes feeds in loose flocks (Hachler, 1959).
The pygmy cormorant (P. pygmeus) is much smaller, less common and extremely restricted in range, although it too feeds largely on fish. Two entirely piscivorous species of pelican, the white pelican (P. onocrotalus) and the dalmatian pelican (P. crispus) occur in southeastern Europe and share similar habitats although the latter is less exclusively found in lowland and coastal areas. Both species exhibit a variety of feeding behaviours, from cooperative to solitary, and feed on a wide range of fishes (Korodi Gal, 1964; Bauer and von Blotzheim, 1986; Brown and Urban, 1969). The dalmation pelican, which is the larger of the two, can take pike (Esox lucius) up to 50 cm in length (Cramp et al., 1977).
Table 8
Summary of principal piscivorous species feeding on European inland water fishes (data taken from Cramp et. al., 1977, 1980, 1983, 1985)
| Species | Distribution | Habitats | Movements | Food |
Red-throated diver (Gavia stellata) | Northern Europe, Finland N. USSR, Norway, Sweden Iceland, Scotland | Small pools, large lakes. Prefers shallow water bodies | Migrating/dispersive. April–September inland. Overwinters on coast | Principally fish (salmonids/roach/dace/bleak) frogs and invertebrates |
Black-throated diver (G. arctica) | Northern Europe, similar to G. stellata | Prefers large, open, deep lakes | Migratory/dispersive. April–September inland. Overwinters on coast | Chiefly fish (perch/trout/bleak/dace/roach/carp) also frogs, insects, snails |
Litte grebe (Tachybaptus ruficollis | Europe, NW Africa, Turkey and Israel. Not further N than S Sweden | Adapted to wide range habitats/lakes/reservoirs/ponds/sewage/canals/rivers/streams | Resident/dispersive/migratory. Generally found inland, migrates coast if winter severe | Fish form 40–50% adult diet in winter and include carp/gudgeon/minnow/roach/dace/rudd/bream bleak/perch |
Great-crested grebe (Podiceps cristatus) | Widely distributed in most of Europe, except for north | Cool-cold standing freshwaters, natural/artificial + fishponds | Migratory/dispersive. Shifts from inland waters-sea in winter | Chiefly fish, including roach/bleak/gudgeon/perch/salmonids |
Cormorant (Phalacrocorax carbo) | Coastal areas of N and W Europe. Also central and SE Europe | West Europe, largely coastal. East Europe/Netherlands breed near large inland waters | Migratory/partially migratory/dispersive, according to population | Almost exclusively fish. In inland waters feed on eel/roach/ruffe/pike-perch/bream /perch/rudd/tench/salmonids |
Pygmy cormo'rant (P. pygmeus) | Restricted range. Occurs small no. in Yugoslavia/Greece/Romania/Turkey | Open standing of slow flowing freshwater/ricefields/swamps/floodlands | Migratory/partially migratory/resident populations | Chiefly fish including rudd/pike/carp/roach/loach/bitterling/tench |
White pelican (Pelecanus onocrotalus) | SE Europe/Romania/Greece/Turkey/USSR | Low-lying/shallow warm water bodies/river deltas/wetlands | Migratory/non-dispersive except in tropics | Almost exclusively fish + roach/carp/bream/rudd/bleak/pike |
Dalmation pelican (P. crispus) | SE Europe, especially Romania/Bulgaria/Albania/Turkey/Yugoslavia/USSR | Similar to often shared with P. onocrotalus also tolerates hilly terrain with small open waters | Migratory/partially migratory | Entirely fish, including carp/perch/asp/roach/pike/tench |
Bittern (Botaurus stellaris) | Widely distributed in E Europe, particularly W. Not in Scandanavia | Lowland swamps, densely vegetated wetlands in middle latitudes <200 m | Partially migratory/dispersive/resident | Chiefly fish (pike/carp/roach/eels/tench) also frogs, mice and insects |
Little bittern (Ixobrychus minutus) | Similar to but more widely distributed than B. stellaris | More adaptable than B. stellaris occupying swamps/rivers/fringes | Migratory/dispersive | Mainly fish (pike/carp/roach/eel) also amphibians and insects |
Night heron (Nycticorax nycticorax) | Patchy distribution throughout middle/south latitudes. In decline in many areas | Warm/temperate/sub-tropical zones, occupying wide range habitats upto 2 000 m including lentic and lotic sites | Migratory/dispersive. Few in Europe from November–February | Chiefly insects, fish (carp/eel/tench/chub) and amphibians |
Squacco heron (Ardeola ralloides) | Very patchy distribution in S. Europe. In decline in many areas | Lowland valleys/wetlands/deltas/estuaries. Prefers pools/ponds/canals/ditches with vegetation | Migratory/dispersive. Overwinter in Africa | Small (10 cm) insects, amphibians, fish (bleak etc.) |
Little egret (Egretta garzetta) | Very patchy distribution S. Europe. Recovering | Usually in lowland shallow lakes/pools/gently flowing rivers. Sometimes coastal areas | Migratory/dispersive. Majority overwinter in Africa | Mainly small (12–15 cm) amphibians, insects, fish (tench/carp/roach/loach) |
Great white egret (E. alba) | Rare, breeding populalations in USSR/Turkey/Hungary/Czechoslovakia | Largely restricted to extensive wetlands and margins of fresh water in lowland regions | Partially migratory/dispersive. Overwinters around Adriatic/Mediterranean | In wet season, chiefly fish (sunfish/carp/tench/trout). In dry season insects |
Grey heron (Ardea cinerea) | Widely distributed mid-latitudes and coastal areas of Scandanavia | Mainly lowland (<500 m) Prefers lotic/lentic shallow fresh waters, with trees close by | Migratory/partially migratory/dispersive. Most European populations overwinter in southern/western areas | Variable. Chiefly fish (1–25 g), amphibians, small mammals |
Purple heron (A. purpurea) | Patchy distributed throughout central and southern Europe | Shallow/eutrophic waters with sand/silt bottom and emergent vegetation | Migratory/dispersive. Most overwinter in Africa south of Sahara | Fishes singly on fish (bream/carp/perch) and insects |
Black stork (Ciconia nigra) | Patchy, in eastern and southern Europe | Undisturbed forest areas with streams or pools; occasionally large lakes | Migratory/dispersive. Overwintering in south Africa/China/India | Chiefly small fish (loach/perch/eel/burbot), also insects, amphibians, reptile |
Smew (Mergus albellus) | USSR. Occasionally overwinter in central and western Europe | Prefers mature woodland near lentic water bodies Occupy ponds/reservoirs | Migratory, although routes little known | Mainly fish (salmon/trout/fry/gudgeon/roach). Insects in summer and Autumn |
Red-breasted merganser (M. serrator) | Widely distributed in N. Europe, especially UK and Scandanavia | Wide range, including oceanic/temperate forest/estuarine. Wintering birds prefer marine habitats | Migratory/partially migratory. Most overwinter in S. Europe | Primarily fish (salmonids/eels/lamprays/minnows/carp) |
Goosander (M. merganser) | Similar to M. serrator | Upper basins of rivers/lakes in forest/mountain areas in close proximity to mature trees | Migratory/partially migratory. Overwinters in NW Europe/Black Sea | Chiefly fish, with some preference for salmonids |
White-tailed eagle (Haligeetus albicilla) | Mainly eastern/coastal/northern Europe including Iceland | Sea coasts/rivers/lakes and wetlands | Resident/migratory/dispersive, according to latitude and age-class | Hunger, scavenger, food pirate, fish (pike/roach/bream/perch), birds, mammals and carrion |
Osprey (Pandion haliaetus) | Eastern and northern Europe/Scotland/Germany. A few Mediterranean populations | Continental areas near clear, unpolluted waters with ample medium-sized fish. Some coastal populations in Mediterranean | Migratory. Overwinter mainly in Ethiopian Africa | Wide range fish (salmonids/pike/perch/road) captured by diving |
Black-headed gull (Larus ridibundus) | Widely distributed throughout N/C/E Europe. Recently in Spain and Italy | Wide range lowland/upper lowland habitats, always near shallow, calm water | Migratory to E and N dispersive/partially migratory elsewhere. | Mainly animal material esp. insects and earthworms. Food pirate and scavenger. Small fish, live/dead shallow wat. |
Common gull (L. canus) | N and E Europe and central western Europe | Generally lowlands continental to coastal avoid frozen and desert conditions | Migratory. Overwinter in W. Europe coastal areas around Black Sea | Chiefly terrestrial/aquatic insects, fish (salmon parr). Direct predation, also food piracy and scavenging |
Lesser black-backed gull (L. fuscus) | Northern Europe (breeding populations) | Prefers flat or sloping sites under short vegetation in temperate and boreal zones, also buildings | Largely migratory. Winter range extends from UK/Black and Caspian Seas/W and E Africa/Mediterranean | Omnivorous. Predation, scavenging and piracy. Fish (roach/perch/bleak/pike/salmon) |
Herring gull (L. argentalus) | Commonest and most widespread of large gulls in W. Paloeartic. Spread throughout W. Europe increasing in range especially inlands | Basically marine, colonies recently estab. inlands, including buildings, lake shores and islands | Migratory in northern Scandinavia and USSR. Elsewhere, resident or dispersive | Predator, food pirate, scavenger. Wide range food, invertebrates, amphibians, fish (roach/perch/pike) and mammals |
Common tern (Strerna hirundo) | Widely distributed throughout coastal areas W. Europe, also continental central and eastern Europe | Along coasts and inland fresh waters in boreal temperate/steppe/semi-desert/Mediterranean. Generally lowland | Migratory throughout most W. Europe. Overwinters western sea-board of Africa, Black and Caspian Seas, Mediterranean | Chiefly marine fish. Freshwater fish include roach/perch/salmonid, etc. |
Little tern S. albitrons) | E. Europe, coastal north, W. Europe, Mediterranean. Few inland populations in W. or central Europe | Frequently coast dwelling, also major rivers and some lakes | Migratory. Many overwinter in Africa | Small fish, including roach, rudd, carp |
Kingfisher (Alcedo atthis) | Widely dispersed throughout Europe except N. Scandinavia and Scotland | Close to clear, ice-free still or gently flowing fresh or brackishwater. Lowlands (<650 m) | Mainly migratory in N. and central USSR/partially migratory in central Europe/dispersive and resident in W. Europe and Mediterranean | Principally small fish (loaches/sticklebacks/minnows, etc.), also insects rarely crustaceans and molluscs |
The family Ardeidae is comprised of bitterns and herons. The bitterns (Botaurus stellaris and Ixobrychus minutus) are only patchily distributed in western Europe, occupying lowland wetlands. They are solitary, crepuscular birds which feed in shallow waters on a wide range of insects, amphibians and fish (Vasvari, 1938; Cramp et al., 1977; Gnetz, 1965; Bauer and von Blotzheim, 1986). The sub-family Ardeinae contains both egrets and herons and includes the little egret (Egretta garzetta) and great white egret (E. alba) and four species of heron (the night heron, Nycticorax nycticorax; the squacco heron, Ardeola ralloides; the grey heron, Ardea cinerea; the purple heron, A. purpurea) which commonly occur in EIFAC member states. All species feed on a wide range of fishes, although the squacco heron and little egret generally only take small (15 cm) individuals (Vasvari, 1938; Cramp et al., 1977; Vasvari, 1954; Dementiev and Gladkov, 1957; Valverde, 1955; Schlegel, 1964; Bauer and von Blotzheim, 1986; Muller, 1984).
Among the storks (Family: Ciconiidae), the black stork (Ciconia nigra) is the most piscivorous, feeding on a wide range of small (9–25 cm) fishes, which it catches by stalking, either singly or in small groups (Dementiev and Gladkov, 1957; Bauer and von Blotzheim, 1986).
A number of wildfowl species, including the smew (Mergus albullus), redbreasted merganser (M. serrator) and goosander (M. merganser) feed chiefly on fish. The smew tends to feed in small flocks and takes a wide range of small fishes, usually 3–6 cm, occasionally 10–11 cm (perch, carp) and rarely up to 29 cm (eel) in length. It modifies its diet in the summer to take advantage of aquatic insects (Dementiev and Gladkhov, 1957; Madsen, 1957; Doornbos, 1979). The meganser and goosander exhibit similar feeding behaviours and have similar dietary preferences. They feed in pair or flocks, often cooperatively, on small (<10 cm) fish (Madsen, 1957; Mills, 1962, 1962a; Nilsson and Nilsson, 1976).
There are three species of fish eagle (Family: Accipitridae, Genus Haliaeetus) which occur in Europe; the African fish eagle (H. vocifer), Pallas' fish eagle (H. leucoryphus) and the white-tailed eagle (H. albicilla).
However, only the latter is common, the other two species being occasional visitors (Cramp et al., 1980). The white-tailed eagle is a very versatile hunter and takes a wide variety of prey, choice being determined by habitat and availability. In some areas fish are taken primarily during the spring and summer months, but account for less than half of the food ingested (Fischer, 1959 in Cramp et al., 1980; Willgohs, 1961; Glutz von Blotzheim, Bauer and Bezzel, 1971; Olsson, 1972), whilst in other areas, fish are taken more often and comprise at least 50% of the food taken at certain times of the year (Kasparsson, 1958 in Cramp et al., 1980; Bergman, 1961). A wide range of fish species is taken, usually in the 05-3.0 kg range, and the most common method of predation is by snatching from the surface.
The osprey (Pandion haliaetus) has a more continental distribution than the white-tailed eagle and feeds almost exclusively on fish which it catches with its talons during shallow (1 m) dives. A wide range of freshwater species are taken and choice is determined by availability, size and behaviour of prey (Curry-Lindal, 1969; Glutz von Blotzheim, Bauer and Bezzel, 1977; Nilsson and Nilsson, 1976; Hakkinen, 1978). Ospreys have also been reported taking fish from carp and trout ponds (Schnure and Thumann, 1961; Deckx, 1978; Mills, 1979).
There are several species of gull (Family: Lariidae), including the black-headed gull (Larus ridibundus), the common gull (L. canus), the lesser black-headed gull (L. fuscus) and the herring gull (L. argentatus), which are increasingly associated with inland, continental sites, and which take fish as part of their diet. Inland populations of black-headed gulls feed on insects and earthworms for much of the year, but will also take fish (including sick and dead individuals), particularly during the winter and early spring months. They generally only take fish from shallow waters, or which are swimming just below the surface, and have been reported feeding at fish ponds (Keve, 1962; Cramp et al., 1983; Mills, 1964, 1980; Harris, 1965; Vernon, 1972). The common gull is regarded as primarily a ground-foraging species (Vernon, 1972), but has occasionally been reported taking fish (e.g., Nilsson and Nilsson, 1976). In a small sample of stomach contents examined between Apirl and June, Mills (1964) found that 53% contained salmon parr. The lesser black-backed gull has been reported feeding on a wide range of freshwater fishes, although there is little quantitative data. Goethe (1975) observed that one Finnish population fed almost exclusively on roach and perch during the summer months.
There have been numerous studies of the diet of herring gull (Cramp et al., 1983). Studies of inland populations in southern Sweden show that a high proportion (50%) of the stomach contents are comprised of freshwater fish (Andersson, 1970; Nilsson and Nilsson, 1976). They have also been recorded at fish farms (Mills, 1979; Ranson, 1982).
Inland populations of two species of tern, the common tern (Sterna hirundo) and little tern (S. albifroms) are widely reported as taking small (2.5–8 cm) freshwater fishes (Bauer, 1965; Lemmetyinen, 1973; Nilsson and Nilsson, 1976).
The kingfisher (Alcedo atthis) is a small, largely piscivorous bird which preys on a wide range of small (usually 3–5 cm) fishes in shallow (1 m) water, either by diving from a perch or by hovering before diving (Hallet, 1977, 1978, 1982; Iribarren and Nevado, 1982).
The above account summarized the principal species of piscivorous birds feeding in European wetlands. However, their economic importance will depend upon the quantities and quality of fish consumed and the intensity of predation at different sites.
The food consumption of birds can either be assessed from field data or from studies of captive birds, and there are disadvantages associated with both methods. The former is usually determined by examination of the gut contents of birds which have been shot, or from regurgitates or from pellets. Birds which are shot often regurgitate food, thus leading to underestimates of fish consumed, whilst it is often difficult to recover regurgitates or pelleted rejecta for analysis. Studies of captive birds are also likely to give underestimates of the quantities of fish consumed since the energy requirement of tree-living birds are some 20–50% higher than for captive birds (Kale, 1965; Wilson and Harmeson, 1973).
Quantitative data on the food consumption of various piscivorous birds are summarized in Table 9. Consumption can be seen to vary almost 100-fold between small birds, such as the kingfisher (average adult weight = 36–46 g) which consumes around 18 g of fish per day, and large birds, such as the white pelican, which can weight up to 11 kg and which can consume 1 600 g of fish in one day. Moreover, smaller birds tend to consume smaller fish which are usually of less economic importance. Thus it would seem that piscivory by the larger species is likely to be of greatest economic importance. However, many of the species isted in Table 9 are unlikely to have a serious impact on commercially important fish stocks or fisheries. For example, the white pelican which nests in large colonies of several hundred thousand and communally feeds on a diet almost exclusively based on medium-sized fish (see above), is comparatively rare, and there are fewer than a dozen colonies mostly sited in Romania, Greece, Turkey and the USSR. Moreover, the species does not tolerate proximity to man and favours areas guarded against disturbance by natural barriers, such as extensive reed beds. Whilst it may well have a major impact on the fishes of certain water bodies, it is unlikely to be a serious competitor with man for these resources.
Table 9
Quantitative estimates of daily food consumption by adult piscivorous birds
| Species | Method of assessment | Amount | References | |
| (g day-1) | (% body wt. day-1) | |||
| Great-crested grebe | Captivity | 150–250 | 20 | Geiger, 1957 |
| Cormorant | Field | 425–700 | 11–17 | van Dobben, 1952; Mills, 1965; Linn and Campbell, 1986 |
| White pelican | Field and captivity | 900–1600 | 9–16 (est.) | Brown and Urban, 1969; Andone et al. in Cramp et al., 1977; Din and Eltringham, 1974 |
| Dalmatian pelican | Field | 1125–1270 | 22–24 (est.) | Dementiev and Gladkov, 1951; Korodi Gal in Cramp et al., 1977 |
| Bittern | Captivity | - | 20 | Lundevall, 1953 |
| Grey heron | Field | 330–500 | 18 (est.) | Creutz, 1958; Junor, 1972; Cook, 1978; Meyer, 1980 |
| Goosander | Captivity | - | 18–27 | Latta and Sherkey, 1966 |
| White-tailed eagle | Field | 500–600 | 9–15 (est.) | Willgohs, 1961 |
| Osprey | Field | 200–400 | - | Schnurre and Thumann, 1961; Nilsson and Nilsson, 1976 |
| Herring gull | Field | 100–200 | - | Spaans, 1971 |
| Pied kingfisher | Field | 18 | 25 | Tjomlid, 1973 |
Other species, such as the bittern, also tend to feed in areas not exploited for fishing by man, such as marshes and swamps, and take small fish of little economic importance.
Some species, such as the osprey and white-tailed eagle, which have a wide distribution, are, nevertheless, comparatively rare. Moreover, they feed singly or in pairs and range over a wide area in search of food and are thus unlikely to seriously deplete the fish stocks of any single water body. This is confirmed by studies conducted at Lake Mockeln, southern Sweden, by Nilsson and Nilsson (1976), who showed that although the osprey was the only avian species whose diet consisted solely of fish, it only accounted for 4.6% of the annual fish consumption by the bird community.
Larger, more valuable, fish tend to be taken by the larger bird species (see above). However, evidence from the literature suggests that birds take very few fish greater than 20 cm (Nilsson and Nilsson, 1976). As recreational and commercial fisheries tend to concentrate on fishes which are larger than this, there is likely to be very little direct competition between birds and man for fish, although predation of small fish by birds may well have a marked impact on recruitment of older year classes (Backiel and Le Cren, 1967).
Species which are likely to have greatest impact on commercially important fishes of European inland waters, in view of the degree of reliance on piscivory, the quantities and quality of fish consumed, their numbers, distribution and feeding behaviour, are the great crested grebe, cormorant, grey heron, merganser and goosander.
Unfortunately, there have been few quantitative studies of the effect of bird predation on fish communities or fisheries. At Lake Mockeln, Sweden, Nilsson and Nilsson (1976) recorded 12 piscivorous bird species and the most important species, in terms of the proportions of fish consumed by the bird community throughout the year, were the goosander (62%), great crested grebe (10%), and grey heron (10%). Fish consumption by birds was estimated to be three times greater than that taken by man from the lake (Figure 2). However, the authors calculated that this was only equivalent to half of the amount that was consumed by the piscivorous fishes.
Most studies of the effects of cormorant predation on fish populations and fisheries of open waters have concluded that it has little, if any, serious long-term impact. Only one study to date, at Lake Constance in Germany, has shown that there may be an adverse economic effect (Deufel, 1984). Van Dobben (1952) concluded that cormorants had only a minor impact on open water fish populations in the Netherlands. More recently, McIntosh (1978) observed large numbers (up to 4.5 km2) feeding and roosting on the lower reaches of the River Tweed in Scotland. However, although it was found that they took considerable quantities of fish (650–700 g/bird/day), mostly small salmonids, it was concluded that predation by the birds was unlikely to significantly affect salmon catches by anglers and netsmen.
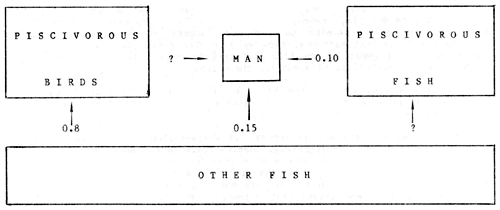
Figure 2 Relationships between fish, birds and man in Lake Mockeln, Sweden. Flow in g/m2/year (redrawn from Nilsson and Nilsson, 1978)
Studies of cormorant predation at open waters in Australia have shown that damage to fisheries is either negligible (Mattingley, 1927; Serventy, 1938; Mack, 1941) or, if it occurs, is localized and transitory in nature (Stead, 1954; Llewellyn 1983). In Lake Malawi, Africa, cormorant predation accounts for 21.63 kg/ha2 of fish production in the southeast arm of the lake, or 20% of the standing crop available prey in the area (Linn and Campbell, 1986). Cormorant consumption was equivalent to 5.5% of the total taken by man from the lake. Moreover, there was very little overlap between the cormorant's diet and the species taken by the commercial fishery, and the authors concluded that this level of avian exploitation could probably be sustained indefinitely by the fish community.
The limited studies of heron predation that have been carried out have also suggested that it has little, if any, impact (Kramer, 1984).
Mergansers and goosanders have been reported by several researchers as having a marked adverse effect on salmon stocks and fisheries (White, 1957; Mills, 1962, 1962a, 1964; Elson, 1962). Whilst Mills clearly demonstrated the importance of salmon parr in the diet of the two species in Scotland, he did not quantify their impact on fisheries. Elson (1962), working on the River Pollett, New Brunswick, Canada, showed that goosander abundance was related to parr density and that greatly increased plantings of hatchery-reared under-yearlings did not result in proportional increases in smolts (Table 10). Since smolt output is related to returns of adult migrating salmon (Huntsman, 1941), it was concluded that the fishery would suffer.
Table 10
Smolt production from known plantings of hatchery-reared under yearling salmon in a 18 km experimental area of the Pollett River Canada (from Elson, 1962; Murton, 1971)
| Year of planting | Number of under-yearlings planted | Parr one year after planting | Total smolts produced from planting | |
| No bird control | ||||
| 1942 | 16 000 | 3 000 | 2 000 | |
| 1943 | 16 000 | 2 000 | 1 000 | |
| 1945 | 249 000 | 12 000 | 5 000 | |
| Control of birds | ||||
| 1947 | 273 000 | 25 000 | 22 000 | |
| 1948 | 235 000 | 45 000 | 14 000 | |
| 1949 | 243 000 | 39 000 | 19 000 | |
| 1950 | 246 000 | 57 000 | 24 000 |
In conclusion, bird predators rarely seem to adversely affect open water fish stocks and fisheries, even when they take comparatively large numbers of fish, unless bird densities are unusually high. From a survey of the literature on factors regulating the density of piscivorous birds, the most important, other than geography, climate, season or time of day, is prey abundance (Elson, 1962; Nilsson and Nilsson, 1976; Mace, 1983; Barlow and Bock, 1984; Eriksson, 1985, 1986). Although the abundance of fish is principally determined by trophic state (Henderson, Ryder and Kudhongania, 1973; Adams, Kimmell and Ploskey, 1983), it can also be affected by pollution, such as acid rain (Eriksson, 1984), and by management. Stocking of open waters with hatcheryreared fishes can dramatically increase the number of avian predators and the consequent predation of fish, as studies in Canada and Australia have clearly demonstrated (Elson, 1962; Mace, 1983; Barlow and Bock, 1984).
Attacks by piscivorous birds on captive fishes have been widely reported from fish farms and hatcheries in Europe and North America (Cottam and Uhler, 1936; Lagler, 1939; Pough, 1941; Scanlon, Helfrich and Stultz, 1978; Mills, 1979; Meyer, 1980, 1981, 1982; Martin, 1982; Marion, 1983; Im and Hafner, 1984; Moerbeek et al., 1987). Indeed, from a survey of 38 fish farms in Scotland, only 13% of respondents claimed that some predation by birds did not occur (Mills, 1979). However, care must be taken to distinguish between birds which visit fish farms and birds which successfully take fish.
Most of the birds reported feeding on open waters have also been observed at fish ponds: the exceptions are those species which avoid proximity to man, such as divers, although others, such as osprey, which is also wary of man, have been recorded fishing at ponds (Schnurre and Thumann, 1961; Deckx, 1978; Mills, 1979). Birds other than piscivores, attracted by the availability of food, have also been reported. These include crows (C. c. cornix, C. c. corone), ducks (A. platyrhynchos) and gulls (L. ridibundus, L. argentatus) (Keve, 1962; Mills, 1979; Ranson, 1982), although the latter are also known to take fish (Mills, 1980).
The most important piscivorous species, in terms of economic damage, are the cormorant and heron. Other species, such as kingfishers and gulls also take fish but, although there are no published data, it is unlikely that they have any serious economic effect in view of the numbers likely to visit farms, their daily food requirements and the sizes of fish that they take (see above).
Heron predation at fish farms has been studied by Hafner and Moser (1980); Meyer (1980, 1981, 1982); Ranson (1982); Ranson and Beveridge (1983); Utschick (1983); Marion (1983); Draulans and van Vessem (1985, 1985a); van Vessem, Draulans and de Bont (1985). These studies show that the number of herons visiting farms tends to increase from February through to August, and then again during the winter months and that birds will travel several kilometres from their nest site/roost site to the farm. Early in the season the majority of visiting birds are breeders, whilst from June onwards, dispersing juveniles predominate. There are two peaks in activity - at dawn and sunset, although visits to water may be restricted to clear nights with good weather. The typical solitary feeding behaviour of the heron is less evident and in excess of 40 birds have been observed at any one farm (Meyer, 1981; Draulans and van Vessem, 1985). Catch rate is highest immediately after arrival and is higher in small rather than large flocks. Fish of 150–250 g are preferred.
From Meyer's work it is apparent that the economic impact of heron predation on pond-based fish farms depends upon farm location, production, pond dimensions and layout, and management. Farms close to heronries are most at risk and well stocked, shallow ponds with gently sloping banks, situated close to trees are most susceptible to predation. At the study farm, Meyer estimated that 4 000 lb of trout consumed by herons between April and August, representing a loss of £3 200, and a further 2% of stock (£130) were rejected for sale due to damage. Studies of economic losses at Danish trout farms have also been made by Moller and Olesen (1984).
Ranson (1982) and Ranson and Beveridge (1983) concluded that few, if any, fish were successfully taken by herons from a cage rainbow trout farm, but they observed that up to 7% of stock bore marks which were attributable to attacks by the birds. The proportion of damaged fish was found to vary with size, mesh size and time spent in the cages.
Studies of cormorant predation have been carried out at a pond-based farm near Lelystad, close to IJsselmeer, the Netherlands (Moerbeek et al., 1987). The farm has 218 ha of ponds which are used to rear both grass (Ctenopharyngodon idella) and common carp. A large colony (approximately 4 500 pairs) of breeding cormorants, first established in 1978, and an additional number of non-breeders, is situated some 13 km from the farm.
Cormorants were observed successfully capturing fish up to 550 g in weight, but were also shown to damage fish of up to 700 g. Losses due to bird predation were estimated by comparison with pre-colony figures and with losses from small, experimental ponds which were carefully protected. Losses during 1970–77 varied between 10 and 25%,. However losses from 1979 onwards, directly attributable to cormorants, were as great as 97% representing a huge economic loss.
In Australia predation of stocked farm dams by cormorants could account for up to 50% of fish (Barlow and Bock, 1984).
A study of cormorant predation at a cage trout farm in Scotland by Ranson (1982) and Ranson and Beveridge (1983) concluded that it was unlikely that the birds were successful in capturing fish, although up to 6% of stock in a cage bore marks consistent with cormorant attack.
In conclusion, it seems that pond-based fish farms can suffer heavy losses from cormorant and heron predation, particularly if the farm is situated close to a breeding or roosting colony. However, it must be borne in mind that the losses incurred at Lelystad (Moerbeek et al., 1987) are unusually high due to close proximity to an exceptionally large breeding colony. Studies of cormorant predation elsewhere (e.g., Im and Hafner, 1984; Deufel, 1984) suggest that losses are usually much lower. Bird predation does not appear to be a particularly serious problem at cage fish farms.
It has been widely observed that there is a relationship between bird species and numbers and the trophic state of a water body. For example, significant correlations between numbers of swans (Cygnus olor) and grebes and trophic state have been reported for Bavarian lakes by Utschick (1976). However, such observations do not necessarily mean that the birds have had any significant effect in determining trophic state.
The role of birds in determining the nutrient status of a water body will depend upon the feeding behaviour, seasonal abundance and community organization of the species. Many species spend a large proportion of their life on a particular water body, nesting and rearing their young and overwintering there, as well as relying upon it as a source of food.
Whilst birds which nest and feed on water bodies, such as grebes and coots, affect nutrient cycling within the water body (Dobrolowski, 1973; Dobrolowski, Halba and Nowicki, 1976) (see below also), birds which congregate in large numbers for extended periods of time and which forage for food outwith the immediate vicinity of the water body are most likely to have a marked effect on nutrient status through the importation of allochthonous materials. Many species of wildfowl (Family: Anatidae) forge for terrestial, as well as aquatic food items (Cramp et al., 1977). Linnman (1983) has speculated that gregarious flocks of migrating shelduck (Tadorna tadorna), whooper swans (Cygnus cygnus), bean geese (Anser fabilis) and greylag geese (A. anser) may cause seasonal influxes of allochthonous materials to lakes around the Swedish coast, thus accelerating eutrophication. However, much of this food comes from around the margins of the water body or from within the watershed and therefore will be of less consequence than food imported from outwith the vicinity.
In view of their social organization, roosting and feeding behaviour, gulls are probably the most important species with respect to influencing trophic state of lakes and reservoirs.
The increase in numbers and spread of Larus spp., principally black-headed, common and herring gulls, which has been widely observed in much of Europe, has been well documented, although the exact reasons remain unclear: increases in the numbers of man-made water bodies (reservoirs, fishponds) and in the availability of food (urban rubbish tips), as well as overcrowding of older roosting/feeding stations have all been suggested (Hickling, 1977; Cramp et al., 1983). Freshwater lentic sites close to urban rubbish tips (Hickling, 1977) or to busy tourist routes, where food at lay-byes is readily available (Jenkins and Bell, 1985), are amongst favoured roost sites for black-headed and herring gulls. Roosting colonies of 10 000 birds are fairly common, and colonies as large as 100 000 are known (e.g., Draycote Water, Warwickshire, UK, Hickling, 1977).
Assessments of the effects of roosting gull populations on nutrient inputs to water bodies have been made by a number of authors (Leentvaar, 1967; McColl and Burger, 1976; Gould, 1977; Gould and Fletcher, 1978; Beveridge, Beveridge and Muir, 1982) and increases in pH, conductivity, organic matter, BOD, nitrogen, phosphorus, coliform bacteria and plankton have all been reported. In terms of trophic state, nitrogen and phosphorus inputs are undoubtedly the most important determinants (OECD, 1982). The daily nitrogen and phosphorus production for a number of species is summarized in Table 11. Unfortunately, the figures for nitorgen loadings are likely to be much higher than shown, as difficulties were experienced in increasing the oxidized inorganic nitrogen components (Gould, 1977).
Annual loadings of nutrients at a site can be estimated using the values in Table 11 and data on colony size, and by invoking assumptions of the duration of foraging gut transit time, distance between feeding ground and roost site, length of time spent at the roost site each day, and the number of days each year that the colony spends at the roost site. Using available published data for a number of water bodies in the UK, areal loadings from roosting gull colonies have been estimated (Table 12). If we assume that the mean depths of these water bodies are between 10 and 20 m (there are no readily available data), and compare the estimated loadings with Vollenweider's (1968) boundary values for oligotrophic and eutrophic lakes, we find that the loadings from birds alone at Draycote Water are sufficient to induce eutrophy, although those for the other two water bodies are likely to be much less significant.
Table 11
Estimated 24-hour nutrient loads (mg) for our species of gull (modified from Gould, 1977; Gould and Fletcher, 1978)
| Species | NH3 | Kj.N | Org.N | Sol.P | Tot.P. |
Herring gull | 402 | 1 819 | 1 416 | 92 | 115 |
Black-headed gull | 211 | 919 | 708 | 47 | 58 |
Lesser black-headed gull | 134 | 829 | 689 | 42 | 50 |
Common gull | 113 | 608 | 495 | 30 | 38 |
Table 12
Estimated1 annual areal loadings of nitrogen and total phosphorous compounds for several UK lakes and reservoirs. Data from Hickling (1977), Benton et al. (1983), Jenkins and Bell (1985), Benton and Khan (pers. comm.), and Sibly (pers. comm.)
| Site | Location | Size (ha) | Colony size | Species | P loading (gm-2y-1) | W loading (gm-2y-1) |
| Draycote Water | Warwickshire, England | 240 (est.) | 90 000 (1973) | Black-headed (60 000) Herring (30 000) | 0.15 | 4.78 |
| Loch Kinnard | Deeside, Scotland | 95 | up to 10 000 | Black-headed gulls | 0.03 | 0.81 |
| Mugdock and Craigmaddie Reservoirs | near Glasgow, Scotland | 61 (total) | 50–420 (1979–86) | Principally herring gulls | 0.002 | 0.083 |
The conclusions drawn above are supported by studies carried out in the USA by McColl and Burger (1976). They demonstrated that 36% of phosphorus inputs to a shallow pool were due to a migrant breeding population of 30 000 Franklin's gulls (L. pipixcan).
Thus allochthonous inputs of nutrients by roosting gull populations can markedly affect the nutrient states of a water body, although the extent or significance of bird-induced eutrophication in European inland waters is difficult to assess. However, in view of the quantities of waste produced per bird, it is likely to be of only marginal importance at all but a few, densely populated open water sites. Moreover, at carp ponds the effects of waterfowl on productivity and fish yields have, of course, been used to great effect (Woynarovich, 1980).