7.1 Salinization
7.2 Salinity
7.3 Crops and saline soils
7.4 Sodicity
7.5 Improvement of saline and sodic soils
7.6 Prevention of salinization
A soil may be rich in salts because the parent rock from which it was formed contains salts. Sea water is another source of salts in low-lying areas along the coast. A very common source of salts in irrigated soils is the irrigation water itself. Most irrigation waters contain some salts.
After irrigation, the water added to the soil is used by the crop or evaporates directly from the moist soil. The salt, however, is left behind in the soil. If not removed, it accumulates in the soil; this process is called salinization (see Fig. 102). Very salty soils are sometimes recognizable by a white layer of dry salt on the soil surface.
Fig. 102. Salinization, caused by salty irrigation water
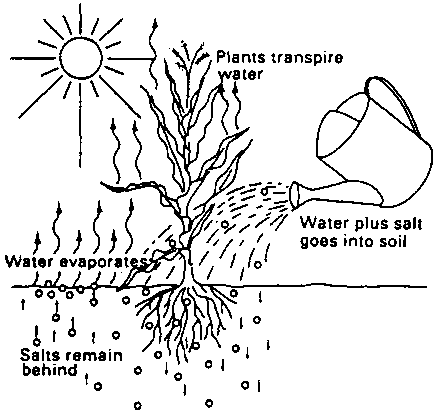
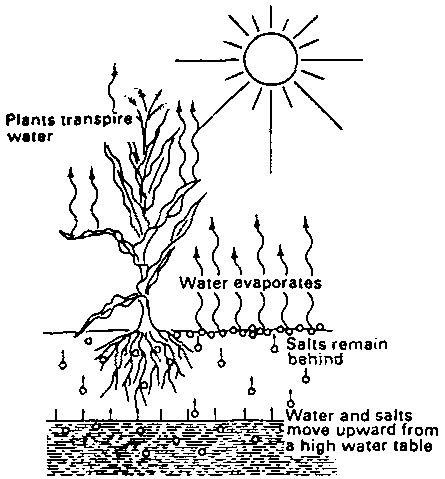
Salty groundwater may also contribute to salinization. When the water table rises (e.g. following irrigation in the absence of proper drainage), the salty groundwater may reach the upper soil layers and, thus, supply salts to the rootzone (see Fig. 103).
Fig. 103. Salinization, caused by a high
Soils that contain a harmful amount of salt are often referred to as salty or saline soils. Soil, or water, that has a high content of salt is said to have a high salinity.
Water salinity is the amount of salt contained in the water. It is also called the "salt concentration" and may be expressed in grams of salt per litre of water (grams/litre or g/l) (see Fig. 104), or in milligrams per litre (which is the same as parts per million, p.p.m). However, the salinity of both water and soil is easily measured by means of an electrical device. It is then expressed in terms of electrical conductivity: millimhos/cm or micromhos/cm. A salt concentration of 1 gram per litre is about 1.5 millimhos/cm. Thus a concentration of 3 grams per litre will be about the same as 4.5 millimhos/cm.
Fig. 104. A salt concentration of 10 g/l
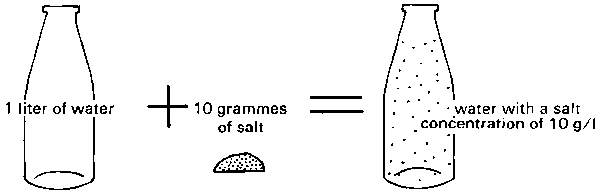
The salt concentration in the water extracted from a saturated soil (called saturation extract) defines the salinity of this soil. If this water contains less than 3 grams of salt per litre, the soil is said to be non saline (see Table below). If the salt concentration of the saturation extract contains more than 12 g/l, the soil is said to be highly saline.
|
Salt concentration of the soil water (saturation extract) |
Salinity |
|
|
in g/l |
in millimhos/cm |
|
|
0 - 3 |
0 - 4.5 |
non saline |
|
3 - 6 |
4.5 - 9 |
slightly saline |
|
6 - 12 |
9 - 18 |
medium saline |
|
more than 12 |
more than 18 |
highly saline |
Most crops do not grow well on soils that contain salts.
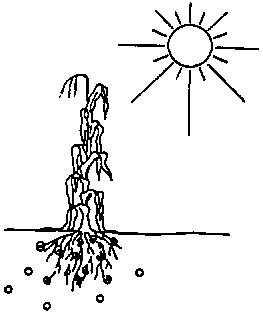
One reason is that salt causes a reduction in the rate and amount of water that the plant roots can take up from the soil (see Fig. 105). Also, some salts are toxic to plants when present in high concentration.
Fig. 105. A high salt concentration in the soil is harmful for the plants as the water uptake is reduced
Some plants are more tolerant to a high salt concentration than others. Some examples are given in the following table:
|
Highly tolerant |
Moderately tolerant |
Sensitive |
|
Date palm |
Wheat |
Red clover |
|
Barley |
Tomato |
Peas |
|
Sugarbeet |
Oats |
Beans |
|
Cotton |
Alfalfa |
Sugarcane |
|
Asparagus |
Rice |
Pear |
|
Spinach |
Maize |
Apple |
|
|
Flax |
Orange |
|
|
Potatoes |
Prune |
|
|
Carrot |
Plum |
|
|
Onion |
Almond |
|
|
Cucumber |
Apricot |
|
|
Pomegranate |
Peach |
|
|
Fig |
|
|
|
Olive |
|
|
|
Grape |
|
The highly tolerant crops can withstand a salt concentration of the saturation extract up to 10 g/l. The moderately tolerant crops can withstand salt concentration up to 5 g/l. The limit of the sensitive group is about 2.5 g/l.
Salty soils usually contain several types of salt. One of these is sodium salt. Where the concentration of sodium salts is high relative to other types of salt, a sodic soil may develop. Sodic soils are characterized by a poor soil structure: they have a low infiltration rate, they are poorly aerated and difficult to cultivate. Thus, sodic soils adversely affect the plants' growth.
7.5.1 Improvement of saline soils
7.5.2 Improvement of sodic soils
Numerous areas in the world are naturally saline or sodic or have become saline due to improper irrigation practices. Crop growth on many of these is poor. However, their productivity can be improved by a number of measures.
Improvement of a saline soil implies the reduction of the salt concentration of the soil to a level that is not harmful to the crops.
To that end, more water is applied to the field than is required for crop growth. This additional water infiltrates into the soil and percolates through the rootzone. During percolation, it takes up part of the salts in the soil and takes these along to deeper soil layers. In fact, the water washes the salts out of the rootzone. This washing process is called leaching (see Fig. 106).
Fig. 106. Leaching of salts
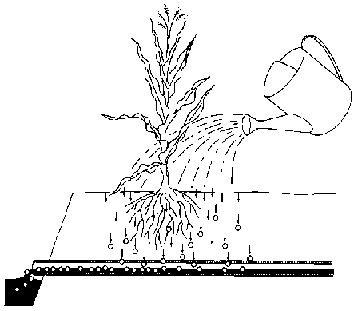
The additional water required for leaching must be removed from the rootzone by means of a subsurface drainage system (Chapter 6). If not removed, it could cause a rise of the groundwater table which would bring the salts back into the rootzone. Thus, improvement of saline soils includes, essentially, leaching and sub-surface drainage.
Improvement of sodic soils implies the reduction of the amount of sodium present in the soil. This is done in two stages. Firstly, chemicals (such as gypsum), which are rich in calcium, are mixed with the soil; the calcium replaces the sodium. Then, the replaced sodium is leached from the rootzone by irrigation water.
7.6.1 Irrigation water quality
7.6.2 Irrigation management and drainage
Soils will become salty if salts are allowed to accumulate. Proper irrigation management and adequate drainage are not only important measures for the improvement of salty soils, they are also essential for the prevention of salinization.
The suitability of water for irrigation depends on the amount and the type of salt the irrigation water contains. The higher the salt concentration of the irrigation water, the greater the risk of salinization. The following Table gives an idea of the risk of salinization:
|
Salt concentration of the irrigation water in g/l |
Soil salinization risk |
Restriction on use |
|
less than 0.5 g/l |
no risk |
no restriction on its use |
|
0.5 - 2 g/l |
slight to moderate risk |
should be used with appropriate water management practices |
|
more than 2 g/l |
high risk |
not generally advised for use unless consulted with specialists |
The type of salt in the irrigation water will influence the risk of developing sodicity: the higher the concentration of sodium present in the irrigation water (particularly compared to other soils), the higher the risk.
Irrigation systems are never fully efficient. Some water is always lost in canals and on the farmers' fields. Part of this seeps into the soil. While this will help leach salt out of the rootzone, it will also contribute to a rise of the water table; a high water table is risky because it may cause the salts to return to the rootzone. Therefore, both the water losses and the water table must be strictly controlled. This requires careful management of the irrigation system and a good subsurface drainage system.