Like other agricultural systems, lagoons can be considered as arenas for production where nutrients and energy are converted to desirable biomass. Production could vary according to either nutrient supply or to the efficiency with which nutrients are converted. The observations by Nixon (1982) that in lagoons: 1) fisheries yield per unit primary production is high; and, 2) primary production is not exceptionally high (but see Correll 1978), suggest that conversion efficiency, not nutrient supply is higher in lagoons than most other aquatic systems. Colombo (1977) suggested that nutrients are rarely limiting in lagoons. Yield, however, is the amount of biomass harvested, and does not necessarily equate to production. Furthermore, Kapetsky (1984) has shown that yield is related to fishing effort. The implication of this is that yield is not necessarily tightly coupled to production, unless one assumes that fishermen in lagoons are exploiting at the maximum production level. Although this is more likely in artisanal fisheries of lagoons than in generally over-capitalized and subsidized ocean fisheries, yield cannot be equated to production or productivity. If conditions for capture are favorable, relatively high yields can be obtained from relatively low production systems, and conversely, low yields can be obtained from high production systems. We are concerned in this chapter with the relationships between hydraulics and production, that is, how much biomass is available to be caught or harvested, not yield. We assume that increasing production will increase yield.
Production (P), the increase in biomass (B) over time, can be represented by the equation:
P = B (G-Z) (3.1)
where G and Z are instantaneous rates of growth and mortality for the time interval of interest. P is expressed as mean weight increase over time if G and Z are ponderal rates. Hydraulics can affect production through B, G or Z in the above equation.
Production of fish or shellfish in lagoons requires: 1) a favorable abiotic environment; 2) food; 3) refuge from predation, and; 4) adequate “seed” or initial numbers (biomass) of immature stages of target organisms. A favorable abiotic environment includes: adequate dissolved oxygen; favorable salinity and temperature regimes; absence of pollutants; and so on. Food must be readily accessible, of the right size, and nutritionally adequate. Refuge from predation refers to either an absence of predators or a means of escape by minimizing encounter, detectability, or vulnerability. Adequate initial biomass is on the one hand obvious, but on the other, rarely cited as potentially limiting in estuarine systems. Indeed, it is easy to find in the estuarine literature either implicit or explicit assumptions of overabundance of larval or juvenile stages. More often than a dearth of colonizing stages, density-dependent growth or mortality is suggested as potentially limiting. This may be the rule in many coastal and oceanic systems, however, the fact that increased yields can be obtained by stocking additional larvae or juveniles in many lagoons (Ardizzone et al. 1988) suggests that the carrying capacity is not exceeded by the numbers which normally colonize these lagoons. We suggest this is more likely the rule than the exception in lagoons. In any case, a determination of the principle limiting factor(s) is critical to effective hydraulic management.
The relationships between the above four requirements and production can be strongly influenced by hydrodynamics, both directly and indirectly. An example of a direct effect is the advection of food to or away from target species. The same currents may increase the turbidity, and thereby affect the feeding efficiency - an indirect effect. In the latter case, to link currents to production one must first link the currents (a vector) to turbidity (a scalar), then link turbidity to feeding, and, finally, feeding to production. In such cases, and there are many, we will point out the scalar to production link(s), where known, then how these scalars are linked to hydraulics, and thus how hydraulics are related to production. The physical links are generally better understood than the physical/biological links. Ultimately, algorithms are needed which relate production to the hydraulics. But since adequate hydraulic models do not exist for most lagoons from which we could derive direct relationships, we necessarily had to adopt a piecemeal approach. In the process we speculate on which of these links, when better understood, will likely be most important.
Perhaps relatively high secondary production in lagoons is to be expected, since lagoons in general display many of the characteristics of aquaculture systems. They are shallow and well-mixed systems, leading to relatively good contact with the atmospheric source of oxygen. Their shallowness tends to encourage benthic productivity, which may mean a more temporally stable supply of food, plus an additional source of primary productivity, compared to more planktonic-based systems. Furthermore, the relatively low diversity of estuarine systems may mean shorter food chains, and the biota of estuaries are reputed to be highly tolerant of adverse abiotic conditions. Estuaries seem to be relatively predator-free, at least when compared to many marine systems, and the biota is often characterized by juvenile stages of long-lived species and adults of short-lived species, both of which exhibit rapid growth rates.
Thus, in many cases, hydraulic management is a question of “fine-tuning” an inherently highly productive system. On the other hand, many lagoons are highly vulnerable to pollution and hypereutrophication - again, like many aquaculture systems. In such cases, hydraulic management is a question of alleviating (or preventing) degradation of the environment. Finally, other lagoons, e.g., in arid regions, may present inherently stressful conditions for aquatic life. In these cases, hydraulic management must be aimed at changing the natural regime into a more benign one. It is clear that different lagoons will require different management strategies, and that production, even under ideal conditions, can be expected to vary widely among lagoons. It is not possible to provide a single “hydraulic prescription” for all cases, and the generalizations presented in this manual must be viewed, and modified, accordingly.
The purpose of this section is to outline some of the more important links between environmental scalars (which could be manipulated hydraulically) and production. The ideal lagoon environment would be maximally hostile to unwanted species, while being maximally benign for target species. Thus, the optimum salinity regime, for example, will vary according to the requirements of the desired species or community. It is assumed that an optimal environment for a lagoon in question can (or will) be detemined in advance of hydraulic manipulation.
It is clear that one of the major sources of poor growth and/or high mortality in lagoons is episodic (or chronic) oxygen depletion. Most often, such problems occur in so-called “dead” zones of a lagoon - regions of little or no mixing - but certain lagoons have a propensity to become anoxic. This is not surprising, because most lagoon sediments, as well as the overlying water, are organically rich and thus have a high potential oxygen demand. Such problems are most likely to occur during hot, still days or nights, especially where high levels of organic or inorganic turbidity exist. Oxygen minima often occur around dawn in response to the combination of nighttime respiration and little or no net production of oxygen. Density stratification exacerbates the problem, both by isolating lower strata from the source of atmospheric oxygen and by increasing resistance to vertical mixing. Clearly, the tendency to become anoxic is proportional to the respiratory demands, thus the more production (primary or above) or the higher the temperature, the greater the risk.
Oxygen depletion is a transitory, and frequently, local, problem in most lagoons. Effects on production can be transitory (temporary reduction in G) or lasting (increase in Z, i.e., decrease in B). but the effects are lasting. And, where lagoons are intensively cultured, the risk is greater. Increased cultural eutrophication will likely increase the frequency of oxygen depletion in many lagoons, even without intensive culture of organisms.
The hydraulics and hydrology of lagoons are linked to oxygen in several ways. First, a major source of dissolved oxygen is the atmosphere, thus, vertical wind-mixing will tend to recharge oxygen-depleted waters. The same wind mixing may increase the contact of overlying waters with sediments, which may have a high oxygen demand in addition to being a potential source of nutrients. High mixing rates tend to resuspend bottom sediments, thereby increasing turbidity, which may decrease primary production. An intermediate rate of mixing apears most favorable. Increased horizontal water movement will tend to alleviate oxygen problems which may develop in certain areas of a lagoon - near aquaculture sites or in relatively quiescent embayments, for example. Indirectly, increased input of freshwater may contribute to oxygen problems by adding nutrients or increasing density stratification, or transporting allochthonous turbidity into the lagoon. Alternatively, increased freshwater input may help alleviate low oxygen conditions by increasing circulation and decreasing mean water residence time and lowering salinity, which lowers the solubility of oxygen in water.
Temperature is most critical for the production of stenothermal organisms. Most estuarine organisms appear to be highly tolerant of temperature variability, and indeed, may gain a competitive advantage over more stenothermal organisms under fluctuating or extreme thermal conditions. Similarly, fluctuating or suboptimal temperatures may provide a refuge from marine predators. The dominance of estuarine biota by a few, seemingly resistant, species supports these hypotheses. Nevertheless, all animals have thermal optima, above and below which growth and survival are reduced. And very large differences in these optima, especially under seasonally covariant thermal and food regimes can produce large differences in growth (Kitchell, Stewart and Weininger 1977). The general relationship between production and temperature peaks at an optimum and falls (more rapidly toward the upper lethal) at higher and lower temperatures. Each species has its own curve which may change ontogenetically, so the optimum temperature for lagoon production will be a compromise.
Excessively rapid temperature changes can be lethal, even within the limits of ultimate upper and lower lethal temperatures for many species. Rapid decreases in temperature are more difficult to tolerate than temperature increases, unless the organism is already near its upper lethal limit.
Temperature interacts with other abiotic factors, e.g. salinity, as well as food. Temperature tolerance changes with salinity in many species. The optimal temperature for growth is lower under limited food conditions.
There is no one best thermal regime for all species or all lagoons, but the above considerations suggest that it should be centered around the joint optimum of the target species and be characterized by some fluctuations. These optima should be determined in advance of hydraulic manipulations, except in the obvious cases where extremes lead to problems of tolerance. In this case, the usual strategy for hydraulic manipulation of temperature should be thermal stabilization - reducing the highs and increasing the lows.
Mean salinity varies both among and within lagoons from near Oppt to greater than full-strength seawater (about 35ppt) depending upon the relative amounts of freshwater and seawater inputs and the rate of evaporation. The importance of salinity to organisms is the difference between the ionic and water concentration in the milieu interieur of the organism and the exterior (lagoon water). Many organisms must spend energy according to this difference in order to maintain their physiological integrity, and this energy could be used for growth or other functions. If the difference or rate of change is great enough the organism is debilitated or dies. The organisms of lagoons are usually quite tolerant of a wide range in salinity (euryhaline). If the salinity of the lagoon is near that of seawater, even stenohaline marine organisms can thrive in lagoons. Usually this is not the case, so the typical estuarine community enjoys, and indeed may depend upon, some degree of isolation from more stenohaline competitors or predators.
Salinity variation is difficult to tolerate since, as with temperature, it requires a continual state of acclimation. The effects of suboptimal salinity and salinity variation on a particular lagoon community are thus difficult to assess, but there are potential positive (decreased competition and predation) and negative (reduced growth and survival) aspects. Organisms typical of lagoon communities seem to benefit more on an ecological level (and suffer less on a physiological level) than more stenohaline marine or freshwater organinsms. In most cases, salinity is less variable than temperature, at least on short time scales.
A final, speculative, aspect of salinity is direct effects upon hormones. Prolactin, thyroxin, growth hormone, thyroid stimulating hormone as well as cortisol are known to respond to salinity in at least some stages of some fishes in a way that enhances somatic growth. Conversely, many estuarine fish appear to need to migrate to sea to spawn. It is possible that the low salinity environment of many lagoons inhibits reproductive maturation, thus enhancing somatic growth. Aquaculturists have appreciated the fact that certain marine fish grow well at low salinity if adequate Ca++ is present. In this (hypothetical) paradigm, salinity is negatively related to somatic growth.
The ideal salinity regime for production would be centered on an optimum range for the target species, but which would include enough variation to suppress colonization (or invasion) by less desirable species. The ideal salinity regime for growth may not be that which minimizes competition or predation. As in the case of temperature, extremes or excessive rates of change must be prevented. If the optimal salinity regime can be defined for the desired community in a lagoon, the hydraulic problem centers around how to maximize the fraction of the lagoon in which this regime exists. The aforementioned horizontal gradients mean that sub-optimal salinities must necessarily exist elsewhere in the lagoon. These latter regions may, however, be vital as refuges when storm episodes, for example, disrupt the “optimal” region.
It must be emphasized at the beginning that the quality and quantity of primary production and its temporal and spatial variation determine the trophic transfer efficiency of a lagoon. Increased primary production of “underutilized” species of algae, for example, can not only divert nutrients from desirable species, but can also increase the risk of oxygen deficits. Controlling the level of primary production, with all of its uncertainty, is easier than controlling the species composition. It is also important to consider that phytoplankton and benthic algae or macrophytes are competing in many cases for the same light and nutrient regime.
Vertical mixing relates to primary production by determining the light environment of phytoplankton and by recharging the euphotic zone with nutrients from either the deeper water layers or the sediments (Legendre 1978, Yentsch 1978). In stagnant, eutrophic waters phytoplankters often accumulate at the surface and become self-limiting. Such conditions also favor the development of blue-green algae, many of which are capable of buoyancy regulation and are nitrogen-fixers. Mixing disrupts this.
The ideal mixing depth is inversely related to the total extinction coefficient, but is always shallow under non-nutrient limiting conditions (Murphy 1962). When the mixing depth exceeds the critical depth (where gross production equals respiration), primary production is depressed because algae spend time in a light regime which is insufficient for net photosynthesis to occur. In actuality, most lagoons are shallower than the critical depth, but high turbidity (either algae or inorganic) can reduce the critical depth to less than a meter. The interplay between critical depth and mixing depth makes a shallow mixing depth essential under turbid conditions. The relationship, where nutrients are not limiting (Murphy 1962), is as follows:
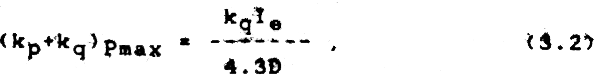
where Ie=incoming solar radiation
kp=extinction by phytoplankton
kq=extinction by inorganic turbidity
Pmax=maximum relative net production
and D=mixing depth.
Increasing the mixing depth from 1 to 2m will reduce Pmax 50% in the presence of 2ppm (kq=1.0) of inorganic turbidity if nutrients are not limiting (Murphy 1962).
If nutrients in the euphotic zone are limiting, however, the effect of mixing will be to recharge the euphotic zone with nutrients. Under these circumstances, the ideal mixing regime for maximum production will thus be alternating periods of deep and shallow (or no) mixing (Ryther 1963, Nihoul 1978). The algal response time is important. Lagoons differ in depth, turbidity and nutrients, therefore the optimum mixing depth and frequency of mixing will also differ.
In nutrient-poor, clear, shallow lagoons primary productivity is shifted toward benthic algae or macrophytes, which have access to sediment nutrients. Even under such circumstances, nutrients in the water column may be important as suggested by the frequent proliferation of macrophytes near inlet streams. Mixing depth is of less consequence under these circumstances. Although macrophytes may provide shelter for organisms, their primary production may be less available to secondary producers than algae. In many aquaculture systems, phytoplankton production is preferred.
The sources of new nutrients to lagoons are the watershed and the ocean, and the atmosphere (Nixon 1982). Excess nutrients from these sources are stored in, and regenerated from, the sediments. The relative importance of different nutrient sources is currently debated, but it is clear that any can potentially control productivity in the short run. The hydraulic implications are different for each. Nutrients are removed from the water column (precipitated) when water movement is minimal and regenerated from the sediments when the mixing depth reaches the bottom. In lakes, prediction of the concentration of a nutrient (N, for example) is as follows:

where L = areal loading rate of N (kg/ha/y)
Z = mean depth
and f = flushing rate.
The term on the right is a correction for nutrient loss to sediments (Vollenweider 1976). The equation suggests that loading with a high volume of low concentration of a nutrient could, by increasing the flushing rate, not only result in a net loss of nutrients, but may also decrease the sedimentation rate.
If nutrients are concentrated in the sediments, however, increasing the flushing rate may increase the rate at which these are resuspended.
To increase productivity of any lagoon, it is first necessary to determine whether nutrient additions (or subtractions) or increases (or decreases) in the rate of recycling are required. Some idea of this can be obtained by short-time scale observations of the lagoon biota to episodic storm events, which often regenerate nutrients from the sediments. In general, increasing either the loading or recycling of nutrients will increase the likelihood of oxygen depletion. Diverting agricultural runoff to a lagoon or increasing the rate of exchange with the ocean will normally result in a decrease in water residence time, but it is clear problems of algal blooms or anoxia more often develop in the vicinity of river mouths than near passes. This may be due to relatively shorter water residence times near passes or the fact that sea water is typically poorer in nutrients than is runoff. At the other extreme, large increases in freshwater inputs may move the familiar zone of precipitation (turbidity maximum) downstream into the lagoon or reduce the water residence time so much that the nutrients are flushed from, rather than cycled in, the lagoon (Flores-Verdugo et al. 1988). The interplay of nutrient loading, sediment recycling, production and hydraulics is best understood in reservoirs, and the reader should consult this literature for principles, bearing in mind that nitrogen, not phosphorous, is usually regarded as limiting in estuarine systems. In larger lagoons, a mosaic of responses to any perturbation is to be expected, owing to spatial variation in local water residence time, etc..
While increasing the primary production in a lagoon increases the algal biomass, it does not necessarily follow that commensurate secondary production will ensue. Besides qualitative shifts, it is important to recognize that zooplankton eat algal biomass, not algal production; carnivores eat zooplankters, not zooplankton production; and so on - apparently as a function of contact rates. Clearly, this depends upon the nature of the predator, but fishes typically feed more efficiently upon aggregations of particles. The structure of the zooplankton community in a lagoon is a complex function of hydraulics - whether their source is exchange with the ocean (marine meso- or holo-plankton) or reproduction within the lagoon (Lam Hoai and Amanieu 1989).
If, for example, increased production is accompanied by increased dispersal or flushing by currents, the availability of food particles may decrease because they no longer occur in patches or are transported out of the lagoon before they can be utilized by consumers. On the other hand, increased turbulence (which may accompany increased flushing) may increase the contacts between predators and their prey, and increased efficiency of feeding may follow (Rothschild and Root 1982). Ideally, the mean consumption rate of food particles should equal or exceed the production and flushing rates. This principle suggests that a relationship should exist between trophic transfer efficiency and hydraulic residence time for a given rate of primary production. At low flushing rates biomass could accumulate faster than it could be consumed leading to the familiar algal bloom conditions. Conversely, at high flushing rates biomass could be removed faster than it could be consumed in a lagoon. A testable hypothesis is, therefore, that maximum transfer efficiency should occur at some intermediate flushing rate approximating the production rate and the consumption rate by the predators.
Thus the optimal current regime/flushing rate is that which jointly optimizes production and consumption. Flushing rates less than this risk blooms, higher rates waste production. Where primary production is high, higher flushing rates are called for; where low, lower rates. In most cases, flushing times of weeks, not days or months, are probably required. Indeed, lagoons may have flushing rates inherently better matched to production rates - one possible explanation for the apparently greater trophic efficiency of lagoons noted by Nixon (1982). Another is suggested in the turbulence/contact rate hypothesis, since many lagoons are relatively well-mixed, but this hypothesis has not been tested.
The hydraulic regime of a lagoon is most directly linked to the distribution of planktonic components of the system. Benthos is not normally advected or dispersed by currents, except in larval stages. For this reason a key feature of lagoons may be the abundance of benthic producers and consumers. Benthic communities and production may also represent more stable sources of food, owing to their capacity to convert detritus and store it (and planktonic production) in a relatively persistent esculent form. The “zone of precipitation” in a lagoon may be especially important in this regard.
However, the less direct links between currents and benthic structure and function are many:
Sessile filter-feeding organisms require advection of food particles.
The hydraulic regime determines deposition rates, thus the food of deposit-feeding benthic organisms.
Mixing rates influence the amount of turbidity, thus the light regime for benthic algal or macrophyte production.
Currents and mixing prevent oxygen depletion in deeper strata (see Section 3.2.1).
Some relationships between currents and benthic structure and production are embraced in the concept of “confinement” (Frisoni, Guelorget and Perthuisot 1984). In theory, regions of a lagoon with relatively long flushing times will be less productive. Similarly, the primary productivities of lagoons which are seasonally isolated from oceans (in dry seasons) is lower than when open (wet seasons) (Kwei 1977). The concept of confinement has not been formalized to the point of quantitative relationships, although it would appear to be an important topic for further investigation.
Predation is implicated as a factor limiting the production of many estuarine organisms. Certainly, if a target organism of either culture or a fishery in a lagoon is the prey of non-harvested species, it is antithetic. Conversely, if the predator is a target species, hydraulic factors affecting its predation (feeding) rate are also of import. The optimal hydraulic regime is that which maximizes feeding by target species, while minimizing predation by non-target species.
The hydraulic regime is linked to predation in two ways. First, the salinity regime of a lagoon determines its habitability by (usually more) stenohaline marine predatory fishes (Loneragan et al. 1986, Miller, et al. 1986). Thus, either a low mean salinity or salinity variability may provide a refuge for prey. Second, turbidity, which is linked to hydraulics, can provide a similar refuge from visually-keyed aquatic and avian predators (Blaber 1980, Whitfield 1983, Bruton 1985). Cyrus (1983) suggested fish entering Natal estuaries were attracted to regions with turbidities greater than 10 NTU; whereas turbidities in excess of 100 NTU apparently discouraged inhabitation. Inorganic turbidity may interfere with filter- or particle-feeding processes. But recently, turbidity has been suggested to enhance the feeding rate of certain estuarine fishes (Boehlert and Morgan 1985). The filtering activities of intensively cultured shellfish may clear the water (G. Lasserre, p.c.). Unfortunately, the hydraulic (turbulence) and turbidity effects have not been separated, so it is unclear in most cases whether it is turbidity, or the turbulence keeping the particles in suspension, that is more important.
Clearly, any change in the hydraulic regime of a lagoon may be expected to alter the predator-prey interactions, thus community structure and production. While the direction and magnitude of change cannot presently be predicted with precision for most lagoon fish communities, it would appear that many lagoon communities thrive at turbidities of 10-100 NTU and salinities of 5-15 ppt - the latter with some variability. Lower turbidities and higher, more stable, salinities invite predation by stenohaline marine fishes. Predation, especially by invading marine fishes, may be episodic, not pervasive, and difficult to detect.
Many of the important species of lagoon fish and shellfish spawn in the ocean, and the larvae or juveniles must migrate some distance to colonize lagoons. Since the young stages have limited vagility, the hydraulic regime can markedly influence the colonization process. As they grow, their capacity to oppose (or select) currents increases (Miller 1988), so a transition from passive to active migration is expected. Three phases of migration are: 1)transport from spawning areas to the lagoon pass; 2)transport through the pass, and; 3)distribution within the lagoon.
Although variation in coastal hydraulics may be a major determinant of migration phase 1, this subject is beyond the scope of this treatise; we will assume the numbers of larvae or juveniles that colonize a lagoon is a function of their entry through the pass.
A present paradigm of larval ecology suggests that excessive numbers of larvae are produced, and they die in a density-dependent fashion. A test of the over-saturation hypothesis is crucial for any management strategy. If excessive numbers enter lagoons, the important relationship between hydraulics and production becomes one of transport and the resulting distribution within the lagoon. If the carrying capacity of the lagoon is not typically reached, its production might be determined by pass hydraulics as well as lagoon circulation.
Thus the two questions we will address in this section are: how does the hydraulic regime in the pass affect the numbers entering the lagoon?; and, how does the hydraulic regime of the lagoon affect the distribution of colonizers? The numbers of colonizers are related to production as a determinant of initial biomass (B) and, if excessive, reductions in growth (G) and mortality (Z) in equation (3.1) may ensue. Their distribution can affect production because a lagoon, unless exceedingly small and well-mixed, is most likely a mosaic of sub-habitats with differing capacities for production (Durand and Skubich 1982, Whitfield 1983, Quignard 1984). The degree to which the lagoon's total productive capacity is reached depends upon whether or not: 1)sufficient numbers of colonizers enter the lagoon; and, 2) their ultimate distribution matches the capacity mosaic.
Since lagoons have restricted channels, which are sometimes closed seasonally, the hydraulic regimes in the vicinity of these channels assume even greater significance than the entrances to drowned river valley estuaries, for example.
The effects of pass configurations on hydraulics were discussed in Section 2.1.6. The effects of passes on the numbers of larval or juvenile fish entering the lagoon depends on the fishes' response and distribution in relation to currents. If migrating by selecting favorable currents, their precision of entry depends upon their ability to locate and remain in flood currents (and/or escape ebb currents). Or, if actively swimming, they must orient upstream to ebb currents and downstream to flood currents (Miller 1988).
Interestingly, this means that passive migrators should be in water of marine origin (since this is most likely to be advected into the lagoon). Buoyancy considerations alone would tend to bias their advection toward the lagoon (Miller 1988). Fish would tend to sink in fresher (ebbing) waters and rise in saltier (flooding) waters. If actively migrating by selecting water masses, the “tidal stream transport” hypothesis (Arnold 1981), they should select the same marine water. If unable to oppose currents, selection of water with “lagoon characteristics” (odor, salinity, temperature, turbidity, etc.) would place them in the water mass least likely to transport them toward the lagoon. However, if actively migrating and opposing currents, they should select water with “lagoon characteristics”. In most cases this means that passive and water mass-selecting fish should be found in the deeper water strata; actively swimming fish near the surface. .bl We can envision three kinds of migrations through passes, each representing a potential increase in precision of entry. First, fish in the vicinity of a lagoon pass could be transported passively into the lagoon. Remaining near the bottom would increase the likelihood in most cases. Second, fish on the bottom (or at the edge) might rise (or move away from the edge) into flooding waters and return to the bottom (or to the edge) on ebbing currents. Third, fish could select and swim against ebbing currents and with flooding currents.
Except for the first instance, the integrity, thus the identifiability, of water masses would facilitate transport. For example, if flooding water were more or less confined to a channel through the pass, or stratified, we would expect a greater precision of entry. Conversely, if water was always mixed vertically, fish might only be able to differentiate flood from ebb flow near the ends of the respective flood and ebb cycles.
Also, it is clear that the difference between the flooding (ocean) water and the ebbing (lagoon) water is important. Thus we might expect to see more efficient migration into, and retention in, lagoons with significant freshwater input. This is somewhat counter-intuitive, since the added tidal asymmetry would seem to increase the probability of fish being flushed out on the (greater) ebb flow. But increased freshwater input also increases the density difference between ebb and flood water, thus the likelihood of stratification. In some cases, there could be a persistent lagoonward current at the bottom of a wide and deep pass similar to that found in drowned river valley estuaries (Dyer 1973).
Once transported through the pass, fish must avoid theebbing water. Advection of flood waters away from the inlet by the residual currents in the lagoon would facilitate this. Furthermore, the same buoyancy response to a lower salinity in the lagoon would tend to trap fish: as they are transported toward the lagoon they generally experience a decline in salinity, which would tend to make them sink. And, if they feed, their sinking rate increases more (Blaxter and Ehrlich 1974), further enhancing the likelihood of retention. In all cases, pass turbulence reduces retention efficiency (van der Veer 1986).
In the lagoon, and beyond the direct influence of tidal exchanges, larvae are distributed according to the wind-driven circulation. In shallow lagoons, these currents can advect fish considerable distances in a short time (see Section 2.2.4). The vertical distribution oflarvae is a critical determinant of their transport vectors, since wind-driven currents tend to flow in opposite directions at the surface and bottom. This generalization is true whether or not the water column is density stratified. If wind stress persists long enough to alter the water level in the lagoon, an upwind-directed return flow will occur in the near-bottom layers (Pietrafesa et al 1986). When the wind stress stops, the return flow occurs throughout the water column. Thus, the resultant distribution of larvae within a lagoon is likely to vary from year to year according to the wind events. Understanding the distribution of larvae in a lagoon requires a three-dimensional (3D) hydrodynamic model of the wind-driven circulation and the recent wind field.
There are few lagoons where the relationship between the wind and current pattern has been investigated and modelled (Millet 1989, Pietrafesa et al 1986). Only in the latter case has the necessary time-dependent larval fish distribution data been obtained to test the model (Pietrafesa et al 1986). In Pamlico Sound (a lagoon), NC, USA, both the temporal and spatial occurrence of fish larvae and juveniles in different peripheral nursery areas were in agreement with model predictions. Millet (1989) found a correlation between the spatial pattern of phytoplankton and the short term hydraulics predicted by his model.
In the case of small lagoons, or lagoons which are colonized by more vagile stages than larvae, distribution of production within the lagoon may be less directly related to the circulation. Indirect effects of the circulation pattern, such as oxygen depletion in certain regions still may lead to a production mosaic.
Redistribution of fish can occur, possibly more or less continuously; for example, in response to depleted food. The “final” distribution is a function of: 1) wind-driven circulation; 2) habitat selection; and, 3) mortality. Again, there are few data from which to derive estimates of the relative importance of these processes. But in nearly every case, spatial variation in production seems the rule, not the exception. Thus, hydraulic management of lagoons should be approached from the perspective of redistributing production as well as that of enhancing overall production.
To enhance the productivity of lagoons with hydraulic manipulations, it is critical to first determine the limiting factor(s). Foremost among these is to assess the adequacy of colonizing stages with respect to the carrying capacity of the lagoon. If there is insufficient colonization, relatively little benefit will acrue with efforts to improve the abiotic environment for growth or survival without also improving the lagoon's ability to entrain and retain colonizing stages of target organisms.
Dissolved oxygen is most often limiting in the bottom layers, and limitation most often occurs during hot, still nights. Such problems occur in vertically stratified lagoons, or regions of lagoons, where resistance to vertical mixing is great, owing to high turbidity or strong vertical salinity gradients. Such conditions prevent recharging the bottom waters with atmospheric or photosynthetically-derived oxygen.
Either too high or too low salinity can stress most lagoon organisms, although many are highly tolerant of salinity extremes. High salinity invites marine predators, which are generally less tolerant of either low, or variable, salinity.
Temperature extremes, particularly low or rapidly declining temperatures often limit growth or survival. Both optimal and sub-optimal thermal regimes exist in many lagoons, and certain regions may serve as thermal refuges during rapid changes. Shallow lagoons are particularly sensitive to thermal changes in the atmosphere, and lethally high temperatures may quickly develop in the surface waters during periods of hot still days. These same meterological conditions also tend to produce oxygen deficits in the lower layers, which ordinarily would serve as thermal refugia.
The above (and other) abiotic factors can interact to either enhance or reduce any or all of the components of the production equation, that is G, Z or B. The magnitude of effects depend upon the organism (s) in question, and such relationships need to be determined for target species in advance of efforts to manipulate the abiotic environment of any lagoon.
The biotic environment for target species consists principally of food and predators. Occasionally disease is a problem. Food must not only be produced, but also must be accessible. High primary production is desirable, except where standing stocks (of algae, for example) lead to oxygen deficits. High planktonic production may also be flushed too rapidly from a lagoon. In general, benthic production is a more stable food source. Macrophyte production is generally less accessable than planktonic production, but may provide an important refuge from predation.