Methods of Water Quality Analysis
Transparency
Fix a bright new pin at the “0” point of a meter scale and dip slowly into the pond water till the pin just disappears from sight. The reading of scale at water surface gives the transparency value.
pH
pH values can be measured directly by a pH meter by dipping the electrode into the pond water or by colorimetric estimation as described below. Initially do the preparatory test with universal indicator to get the approximate value of the pH. Place 10 ml of the water sample in the glass tube provided with the colour comparater and add 0.2 ml of universal indicator. Shake gently and match the colour against standard colour disc for that indicator. After ascertaining the approximate pH value use suitable indicators to determine the exact pH. Bromothymol blue for pH range of 6.0–7.6, phenol red for 6.8–8.4 and thymol blue for 8.0–9.6 should be used as indicators. After adding the required indictor stirr the sample and match the colour against appropriate standard colour disc and read the values.
Alkalinity
Reagents and equipments required:
0.02(N)H2SO4 - Dilute 30.0 ml of conc.H2S04 (S. gravity 1.84) with distilled water to make 1 l to get approximately 1(N) stock solutions. Take 20 ml of this solution and further dilute it to make 1 l to get 0.02(N) solution. Check it against 0.02(N) Na2CO3 using methyl orange indicator.
Phenolphthalein indicator - 0.5% solution in 50% alcohol
Standard 0.02(N) Na2CO3 - Dissolve 5.3 g anhydrous and dessicated Na2CO3 in 1 l distilled water to make 0.l(N) Na2CO3 stock solution. Dilute 50 ml of this solution to make 250 ml to give 0.02(N) Na2CO3.
Methyl orange indicator - 0.05% aquous solution,
Glasswares
Procedure:
Phenolphthalein alkalinity (P)
Take 50 ml of the sample in white porcelain basin and add 2 drops of phenolphthalein indicator. If the sample remains colourless (P) alkalinity is zero, but if it turns pink, titrate with 0.02 (N)H2SO4 through a burette to a colourless end point and calculate the value as per the following equation.
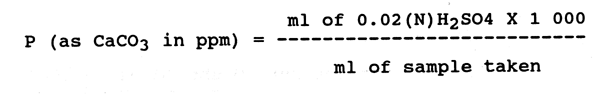
Methyl orange alkalinity (M):
Proceed as above using methyl orange as indicator, the
end point is indicated by a colour change from yellow to
faint orange.
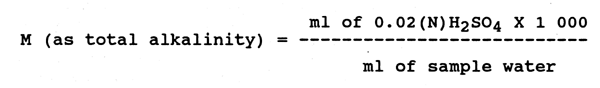
Dissolved Oxygen (DO) (Winkler's method):
Alkaline iodide: Dissolve 700 g of pure potassium hydroxide (KOH) and 150 g of potassium iodide (KI) or 135 g of sodium iodide (NaI) in 800 ml of distilled water. Cool it and make it to 1.0 1 by adding more distilled water.
Manganous sulphate - Add 480 g of managanous sulphate (MnSO4.4H20 or 400 g of MnSO4.2H20) in 250 ml of distilled water, mix well and add more water to make the solution upto 1 l mark.
Concentrated H2SO4 - (Sp. gravity 1.84)
0.025(N) Sodium thiosulphate - Dissolve 24.82 g of crystalline sodium thiosulphate (Na2S2O3.5H2O)in 700 ml of distilled water and add 4 g of borax (Na2B4O7.10H2O). Add more distilled water to make 1 l; after borax is dissolved.
0.025(N) Potassium dichromate solution - Take 1.226 g of potassium dichromate (K2Cr2O7) and dissolve in 1 l distilled water.
Starch solution - Take 1 g of starch powder in 5 ml of cool distilled water, mix well and add 100 cc of boiled distilled water. Add 3 g of boric acid as preservative.
Glasswares: Take 10.0 ml of 0.025 (N) K2Cr2O7 in a conical flask and add 1 ml of alkaline iodide, 2 ml of Conc.H2SO4 and titrate with 0.025(N) Na2S2O3 solution using starch as indicator.
Procedure:
Water samples for DO should be collected in 100 ml DO sample bottles without agitating, bubbling or mixing with air from the top column or bottom layer of the pond water as required. Immediately after collection, carefully remove the stopper and add 1 ml each of reagent (i) and (ii) by 1 ml pipette. Replace the stopper and thoroughly mix the contents. A whitish to deep brownish precipitate will be formed which will settle at the bottom. Whitish colour indicates poor DO level while more deeper the colour of the precipitate higher the DO level. Brown to red brown colour indicates medium to high DO concentration. Add 2.0 ml of conc. H2SO4 to dissolve the precipitate. Take 50 ml of this solution and titrate with 0.025 (N) Na2S2O3 using starch as indicator to the colourless end point.
Calculation:
Dissolve oxygen (ppm) = ml of 0.025 (N) Na2S2O3 used X 4.
Dissolved free carbon dioxide:
Reagents and equipments required:
N÷44 Sodium carbonate (Na2CO3) - Dissolve 5.3 g Na2CO3 in 1 000 ml of distilled water. Dilute 100 cc of this solution (N/10) to 440 ml with distilled water to get N/44 Na2CO3.
Phenolphthalein indicator.
Procedure:
Take 50 ml of the sample in a conical flask and add 2 drops of phenolphthalein reagent. If the water turns pink there is no free carbon dioxide, if not, add N÷44 Na2CO3 drop by drop from a 10 ml graduated pipette with simultaneous gentle stirring with a glass rod till the colour turns pink.
Calculation:
Free carbon dioxide (ppm)
= ml of N÷44 Na2CO3 required × 20
Nitrogen (Ammonia and Nitrate nitrogen)
Reagents required:
Nessler's solution: dissolve 545.5 g of A.R. grade mercuric iodide and 35.0 g of potassium iodide (KI) in limited volume of ammonia free distilled water and finally add this mixture slowly to a cold solution of 112.0 g potassium hydroxide (KOH) dissolved in 500 ml of ammonia free distilled water. Dilute to 1 l and allow to stand for few days and finally the supernatant liquid is decanted off into dark coloured bottle and kept for use.
Devarda's alloy
Magnesium oxide (MgO)
Ammonia free distilled water
Kjeldahl flask and other laboratory glasswares
Procedure:
Take 100 ml of filtered water sample in Kjeldahl flask and fit the flask with distillation unit. Add about 1 g MgO and start distillation. Continue distillation till all the NH4-N distilled off. Collect 25 ml of the distillate. This contains NH4-N.
Add 1 g of Devarda's alloy to the remaining sample of the flask and start distillation again. Collect 25 ml of the distillate in a separate receiver flask. This fraction of distillation contains NO3-N.
Place both the distillates in two separate Nessler tubes and add 0.5 ml Nesseler's reagent in each. Mix the solution and match the developed colour against standard colour discs for ammonia and nitrate after 10–15 minutes with a Nessleniser (BDH Nessleniser).
Calculation:
Amount of Ammonia/Nitrate Nitrogen (ppm)
= Number of matching division of the standard disc × 10 × 0.001 (Standard of each disc division).
Dissolved Inorganic Phosphorus
Reagents required:
2.5% sulphomolybdic acid - Dissolve 25 g pure ammonium molybdate in 1 200 ml distilled water by warming at 60°C. Dilute 275 ml of concentrated sulphuric acid to 750 ml with distilled water separately. After cooling slowly mix ammonium molybdate solution to the diluted H2SO4 with constant stirring. Make the volume up to 1 l by adding more distilled water and store in dark bottles.
2.5% Stanous chloride - Dissolve 2.5 g of stannous chloride (SnCl2.2H2O) in about 5 ml of concentrated HCl with little warming. Dilute to 50 ml with freshly boiled distilled water and finally make the volume up to 100 ml by adding 1.2(N) HC1. Preserve the reagent in dark bottle by overlaying a thin layer of pure liquid paraffin.
Procedure:
Take 50 ml of filtered water sample in a Nessler tube and add 2 ml of sulpho-molybdic acid and 5 drops of stannous chloride solution. Mix thoroughly and compare the developed blue colour after 3–4 minutes in a Nessleniser using standard colour discs for phosphate.
Calculation:
Phosphate (P2O5) in ppm
= Disc reading for 50 ml × 2 × .01
Dissolved Organic Matter
Reagents required:
Standard KMnO4 solution (1 ml = 0.1 mg O2) - Dissolve 0.4 g potassium permanganate (KMnO4;) in distilled water and make up to 1 l. One ml of this solution = 0.1 mg O2- This solution should be standardised against ammonium oxalate solution in acid medium so that 1 ml of KMnO4= 1 ml of ammonium oxalate.
Standard Ammonium Oxalate solution - Dissolve 0.888 g ammonium oxalate in distilled water and make up to 1 l. (1 ml of this solution = 0.1 mg of O2).
Dilute sulphuric acid (1:3) - Add 100 ml of concentrated sulphuric acid slowly into 300 ml of distilled water.
Procedure:
Place 50 ml of the filtered sample water in a 250 ml conical flask and acidify by adding 5 ml of dilute H2SO4. Add 10 ml of standard KMnO4 solution and keep on water bath for half an hour. After removing from water bath add 10 ml of ammonium-oxalate solution. The pink colour of permanganate will disappear. With the help of a 10 ml graduated pipette add drop by drop standard KMnO4solution till the colour just reappears. At times the pink colour disappears while heating on water bath itself, in such cases 20 ml or more KMnO4 solution is to be added.
Calculation:
Oxygen consumed (ppm) = No of ml potassium permanganate required × 0.1 × 20.
APPENDIX II
Methods of Soil Quality Analysis
Minimum five samples should be collected from a larger pond from soft layer. However, the number of samples depend upon the variability of the soil quality. Mix all the samples well to get uniform composite sample. Samples should be air dried in shade and ground to fine powder by wooden hammer and strained through 2 mm sieve.
Soil Texture
1.1 Gravimetric method Required reagents:
Hydrogent peroxide (6.0%)
(N) Hydrochloric acid
(N) NaOH solution
Silver nitrate solution (5%)
Concentrated Ammonium hydroxide solution
Procedure :
Take 20 g soil in a 400 ml beaker, add 35 ml H2O2 while keeping the beaker on water bath. Add more H2O2 when the reaction is over till no more frothing takes place. Cool and add 50 ml (N) MCl and 200 ml distilled water. Allow the content to stand for an hour with occasional stirring. Filter the soil and wash free of acid with hot water, tested by AgNO3 solution. Transfer the soil to 1 l beaker, add 8 ml (N)NaOH solution and shake for 20 minutes in a mechanical shaker. The contents now should be transferred to a 1 000 ml measuring cylinder, shake vigorously for 1 minute and allow to stand for 4 minutes. Suck 20 ml of the content with a 20 ml pipette from 10 cm level. Dry it in a beaker till constant weight is attained which gives the weight of silt and clay. Repeat the operation after 6 hours to get the weight of clay alone. The percentage of sand is obtained by deducting percentage of clay + silt from 100, similarly percentage of clay is substracted from that of clay + silt to get the percentage of silt.
1.2 Hydrometer method
Reagent required:
Sodium oxalate (COONa)2 0.5(N} solution - Dissolve 33.5 g sodium oxalate in 1 l of distilled water.
Procedure:
Place 100 g of air-dried finely powdered soil in a 500 ml conical flask and add 15 ml of 0.5(N) sodium oxalate. Add 200 ml of distilled water and shake for 20 minutes. Transfer the content to 1 l capacity measuring cylinder and make it up to 1 l mark by adding enough water. Stir the suspension thoroughly, stop stirring and note the time. Dip the hydrometer in the suspension after 5 minutes which will give direct reading of the percentage of clay + silt. Hydrometer reading after 5 hours of sedimentation and the temperature of the suspension gives the percentage of clay directly.
Calculation:
Hydrometer gives the reading in g/1 which can be converted easily into percentage of suspended matter. Percentage of sand is determined by deducting the percentage of clay + silt from 100. Similarly percentage of silt is determined by substracting the hydrometer reading for clay from the silt + clay. Normally the hydrometer reading is adjusted for the temperature of 19.4°C. Make correction to the scale reading by adding 0.3 units for every degree of temperature above 19.4°C or substracting 0.3 units for each degree below 19.4°C.
pH
2.1 Colorimetric method
Reagents required:
Neutral Barium Sulphate (A.R. grade)
Indicator solutions - see Section 2 of Appendix I. Place a layer of neutral barium sulphate 1 cm thick in a 50 ml dry test tube, add 10 g of air-dried powdered soil sample and add 25 ml of distilled water, shake well and keep it for settling. Take 10 ml of clear aliquot and follow the same procedure as under Section 2 of Appendix I.
2.2 Potentiometric method
Take 10 g of air-dried finely powdered soil sample in a beaker and mix well with 25 ml of distilled water and keep for about half an hour with occasional stirring. Dip the electrodes/electrode of pH meter into it and take the reading directly.
Organic Carbon
Reagents required:
(N) Potassium dichromate solution. Add 49.04 g A.R. grade potassium dichromate (K2Cr2O7) in distilled water-to make it 1 l.
(N) Ferrous solution. Dissolve either 278.0 g of A.R. grade ferrous sulphate (FeSO4.7H2O) or 392.13 g of ferrous ammonium sulphate (FeSO4. (NH4)2 SO4.6H2O) in distilled water, add 15 ml of conc. H2SO4 and make the volume to 1 l. Standardise against (N)K2Cr2O7 so that 1 ml of FeSO4 solution = 1 ml (N)K2Cr2O7 solution.
Diphenyl amine indicator - Dissolve 0.5 g of diphenylamine in 10 ml conc.H2SO4 and 20 ml distilled water.
Phosphoric acid (85%)
Conc. sulphuric acid (sp.gr. 1.84).
Procedure:
Place 1 g of soil sample (0.5 g and 2.0 g for soils with expected high and low organic C levels respectively) in a 500 ml flask. Add 10 ml of reagent (i) and mix thoroughly. Add 20 ml of reagent (v) and mix gently by rotation. Allow the mixture to stand for 30 min. Add water to make up to 200 ml and then add 10 ml of reagent number (iv). Titrate with (N) Fe(NH4)2SO4 or (N)FeSO4 solution using 1 ml diphenylamine as indicator. At the end point colour of the solution sharply changes to a brilliant green. Carry out a separate standardisation blank also using all the reagents except the soil sample.
Calculation:
Organic carbon (%) = (Titration value (ml) for blank-titration value(ml) with soil) × 0.3
Total Nitrogen
Reagents required:
Concentrated sulphuric acid (A.R. grade sp.gr. 1.84)
Salicylic acid (A.R. grade)
Sodium thiosulphate (Na2S2O3.5H2O)
12(N) Sodium hydroxide - Dissolve 480 g of sodium hydroxide (NaOH) pellets in distilled water and make up to 1 l.
0.1(N) NaOH - Dissolve 4 g of NaOH pellets in distilled water, make up to 1 l and standardise against 0.1(N)H2SO4.
0.1(N) Sulphuric acid (H2SO4) - Dilute 100 ml of (N) H2SO4 (stock solution - preparation under Section 3 of Appendix I) to 1 l and standardise against 0.1(N) Na2CO3 solution.
0.1(N) Sodium carbonate (Na2CO3) solution. Dissolve 5.3 g of Na2CO3 in 1 l of water to get 0.1(N) standard solution.
Copper sulphate
Potassium sulphate
Procedure:
Take 10 g of air-dried soil in a Kjeldahl's flask. Add 30 ml of conc. H2SO4, 1.0 g of salicylic acid, and keep in cold for 1/2 hour. Now add 5.0 g of sodium thiosulphate and again keep for 1/2 hour. Add 1.0 g of powdered copper sulphate and 10.0 g of potassium sulphate and digest the mixture. Clear blue colour of the solution indicates completion of digestion. Cool and transfer with water to an ammonia distillation flask. Make it alkaline with excess of 12(N)NaOH using phenolphthalein as indicator and distill off the ammonia collecting it in 25 ml of 0.1(N)H2SO4 in a conical flask with a few drops of methyl red indicator. Collect about 150 ml of the distillate. Titrate the excess of 0.1(N)H2SO4 with 0.1(N)NaOH till the solution turns colourless.
Calculation:
Total nitrogen (%)
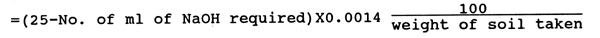
Available Nitrogen
Reagents required:
.02(N) sulphuric acid - see Section 3 of Appendix I
0.02 N sodium hydroxide - Dilute 100 ml of 0.1(N) sodium hydroxide (NaOH) of standard stock solution to 500 ml with distilled water (Section 4 of this Appendix)
Methyl red indicator - Dissolve 0.1 g of methyl red powder in 25 ml ethyl alcohol and make up to 50 ml with distilled water.
0.32% potassium permanganate (KMnO4) - Dissolve 3.2 g of KMnO4 in distilled water and make the volume up to 1 l.
2.5% sodium hydroxide - Dissolve 25 g of NaOH pellet in 1 l of distilled water.
Procedure:
Take 10 g of air-dried and powdered soil sample in a Kjeldahl's flask. Add 100 ml of 0.32% KMnO4 and 100 ml of 2.5% NaOH solutions. Distill the mixture after adding 2 ml of liquid paraffin and 10–15 ml of glass beads. Collect 75 ml of the distillate in the receiving flask containing 25 ml of 0.02 (N)H2SO4 with a few drops of methyl red indicator and titrate with 0.02 (N) NaOH to a colourless and point.
Calculation:
Available nitrogen (mg/100 g soil) = (25 - No. of ml of 0.02 (N)NaOH required) × 2.8
Available Phosphorus
Reagents required:
2.5% Sulphomolybdic acid (see Section 7 of Appendix I)
2.5% Stannous chloride (SnCl2) (see Section 7 of Appendix I)
0.002 (N) H2S04 - Dilute 50 ml of 0.02(N) H2SO4 (Section 3 of Appendix I) with distilled water to make up to 500 ml mark and adjust the pH to 3.0 with (NH4)2 SO4 or K2SO4 (approximately 3 g/l)
Standard phosphate solution
Dissolve 0.2195 g of dried monobasic potassium dihydrogen orthophosphate in 400 ml of water. Add 25 ml of H2SO4 (water mixture 1:5) and make the volume up to 1 l with addition of distilled water. This will give a stock solution of 50 ppm of P (Phosphorus). Dilute 20 ml of this solution to 500 ml to get 2 ppm solution of P. This 2 ppm of P solution, when diluted to 50 ml volume for the development of phospho-mollybdic blue colour, gives the following values under different concentrations.
Procedure:
Standard curve: Take 0.5 ml, 1.0 ml 2.5 ml, 5.0 ml and 10.0 ml of 2 ppm solution of P in 50 ml capacity volumetric flasks. Add 2.0 ml of sulphomolybdic acid in each. Make the volume up to 50 ml mark by adding distilled water and add 5 drops of SnCl2 while shaking gently. The colour develops at its full intensity in 3–4 minutes and begins to fade after 10– 12 minutes. Find out the respective optical density readings by the help of a photoelectric colorimeter or a spectrophomometer and plot the readings against the corresponding concentrations of P to prepare a standard curve.
Take 1 g of air-dried and powdered soil sample in a glass bottle with stopper, add 200 ml of 0.002 (N) H2SO4 solution and shake for 30 minutes with a mechanical shaker. Filter the suspension immediately on a Whatman No. 42 filter paper. Take 25 ml of the clear filtrate and find out the concentration of P in that solution through the standard curve.
Calculation:
Available P mg/100 g soil = ppm P in solution × 20.
APPENDIX III
Methods of Analysis of Feed and Feed Ingredients
Moisture
Requirements:
Petridish Drying oven Balance
Procedure:
Place pre-weighed 4–5 g of the sample in a covered petridish and dry at 100–105°C in a drying oven till constant weight is achieved.
Calculation:
Moisture content (%)
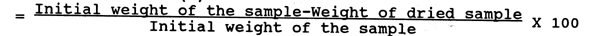
Crude Protein
Requirements:
Concentrated Sulphuric acid (A.R. grade - nitrogen free)
Potassium sulphate (A.R. grade)
Mercuric oxide (A.R. grade)
Paraffin wax
Sodium hydroxide solution (40%) - Dissolve 40 g of NaOH pellets in 100 ml of distilled water.
Sodium sulphide solution (4%) - Dissolve 4 g of sodium sulphide in 100 ml distilled water.
Pumic chips
Boric acid/indicator solution - Add 5 ml of indicator solution (0.1% methyl red and 0.2% bromocresol green in alcohol) to 1 l of saturated boric acid solution.
Hydrochloric acid (0.1 N) - Dissolve 1.16 ml of concentrated A.R. grade HCl with distilled water to make 1 l.
Kjeldahl digestion and distillation units
Kjeldahl flasks (500 ml cap)
Conical flasks - 250 ml
Procedure:
Take exactly 1.0 g of sample into the Kjeldahl flask and add 10 g digestion mixture which consists of potassium sulphate and copper sulphate in 9:1 ratio and 20 ml of sulphuric acid. Heat the flask gently at an tilted position till frothing stops and then boil until the solution becomes clear. To control excessive frothing add a small amount of paraffin wax. Cool and add 90 ml of distilled water, leave it for some time and add again 25 ml of sulphuric acid and mix. To prevent bumping put small piece of boiling chips and add 80 ml of sodium hydroxide (NaOH) solution while tilting the flask so that two layers are formed. Connect rapidly to the condenser unit, heat and collect distilled ammonia in 50 ml boric acid/indicator solution. Collect the distillate. On completion of distillation, remove the received and wash condenser tip and titrate against 0.1(N) HCl.
Calculation:
Nitrogen content of sample (%)
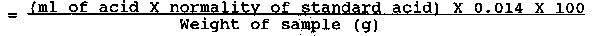
Crude protein (%) = Nitrogen content × 6.25
If you suspect mixing of urea in the sample, then wash the sample thoroughly with distilled water and dry at 60°C before proceeding for protein estimation.
Crude Fat
Requirements:
Petroleum ether (B.P. 40–60°C) Extraction thimbles/flasks Soxhlet extraction apparatus
Procedure:
Take 2.3 g of dried sample either in an extraction thimble or in a silk bag. Place the thimbles or the bag inside the soxhlet apparatus/soxhlet flask. Connect a dry pre-weighed solvent flask beneath the apparatus and add the required quantity of solvent and connect to the condenser. Adjust the heating rate to give a condensation rate of 2–3 drops/ second and continue extraction for 16 hours. By increasing the extraction rate the extraction time may be reduced. On completion remove the thimble. Remove ether completely on a boiling bath and then dry the flask at 105°C for 30 minutes. Cool the same in a desicator and weigh.
Calculation:
Weight of the crude fat = Final weight of the solvent flask = Initial weight of the solvent flask.
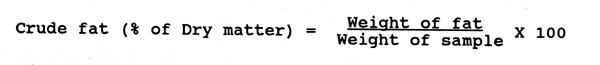
If the extraction is done by putting the material in pre-weighed silk bags and hanging in extraction flask then follow the following calculation.
Weight of the crude fat = Initial weight of the bag with material - Final weight of the bag with the remaining material.
Carbohydrate
Requirements :
Standard glucose solution - 100 mg in 100 ml of distilled water.
Benedict's reagent
6(N)Hcl - Dilute 69.6 ml of concentrated HCl (A.R. grade) with distilled water to make 1 l volume
Sodium carbonate
Procedure:
Take 100 mg of powdered sample and dissolve in 25 ml of water, add 25 ml of 6(N)HCl and heat in a water bath for 3 hours at 100°C. Cool and neutralise with sodium carbonate until frothing stops and centrifuge the solution at 2 000 rpm for 10 minutes or filter. Take the supernatant or filtrate and make upto 100 ml by taking enough distilled water.
Take 5 ml of Benedict's solution in a conical flask, add 1 g of sodium carbonate and put some glass beads and titrate against standard glucose solution. The titration must be done only in heated condition. Now the same volume of Benedict's reagent is titrated against the hydrolysed sample solution.
Calculation:
Volume of standard glucose solution required for 5 ml of Benedict's reagent = A.
Volume of hydrolysed solution required for 5 ml of Benedict's reagent = B.

Ash
Concentrated Nitric acid
Silica crucible
Muffle furnace
Procedure:
Take 2 g of sample in a clean, dry silica crucible and place in a muffle furnace at 600°C for 6 hours. Cool and add 2 drops of concentrated nitric acid. Again put the sample in muffle furnace and heat till white ash is produced. Cool the crucble in the desicator and take the weight.
Calculation:
Weight of the crucible - Ag
Weight of the crucible + sample - B g
Weight of the sample - B-A = Cg
Weight of the crucible + ash - Dg
Weight of ash - D-A = Eg
APPENDIX IV
Methods of Community Structure Analysis
1. Plankton Analysis
Information on the abundance and variations of natural fish food organisms is necessary for proper fishery management. Methods of plankton analysis include collection of plankton samples and analysis of the samples both quantitatively and qualitatively.
1.1 Collection of samples
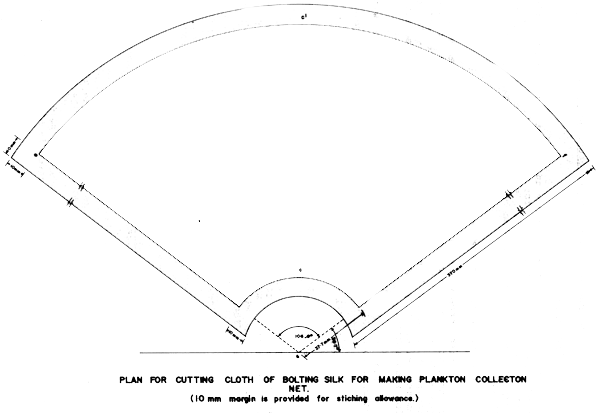
In fish ponds plankton samples are generally collected using a truncated cone shaped net by filtering known volume of water (normally 50 or 100 1). The plankton sieving net is the common equipment used and is made of bolting silk cloth No. 25 (# 0.064 mm mesh size) for phytoplankton and No. 13 (# 0.112 mm mesh size) for zooplankton.
The plankton cloth is cut based on the following calculations.

Using 1+X as radius, lay off the arc C on a piece of paper. At Centre h, lay off angle a by means of a protractor and draw lines he and hf. With x as radius, draw arc C of smaller circle. Leaving 1 cm all along the sides, the cloth may be cut and stitched and fitted onto a brass frame having wooden handle.
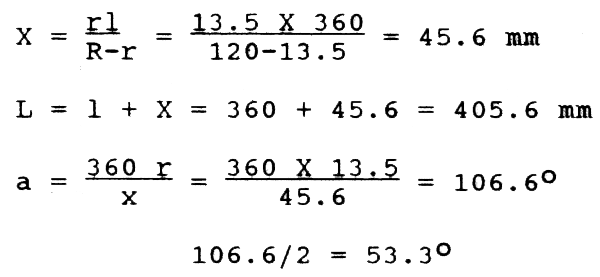
For he and hf, mark points at 90 + 53.3 = 143.3° and 90 - 53.3 = 36.7°
Usually about 50–100 1 of water is filtered through the plankton net and the sample is preserved in 5% formaldehyde. In the laboratory, the preserved plankton samples are analysed for quantitative and qualitative aspects.
1.2 Quantitative analysis of total plankton:
Settling volume:
Transfer the sample to a graduated cylinder or centrifuge tube and allow sufficient time (at least 6–8 hours) for plankton to settle at the bottom and record its volume and express the volume as ml of plankton/1 or ml of plankton/m3. Centrifuge of the samples may also be resorted to, for quicker analysis.
Wet weight:
The plankton sample is filtered through bolting silk cloth, excess water is blotted out and the residual material is weighed. The wet weight is expressed as mg/1 or g/m3 water.
Dry weight:
After taking the wet weight, dry the plankton samples in a hot-air oven at 60–80°C for about six hours and take the weight on a sensitive balance. Express the weight as mg/1 or g/m3.
Numerical count:
Dilute the filtered sample to a known volume, say 10 ml, and take for counting under microscope. Shake well the diluted plankton sample and take one drop for counting on a glass slide and cover with a cover slip or take 1 ml of plankton suspension in the Sedgewick- Rafter counting cell having a capacity of 1 ml with its area divided into 1 000 equal squares. Count the number of plankters under microscope with 10x and 10x lenses. If 100 squares at random are counted, and 100 1 water had been filtered, the number per litre will be given by X × 10 × 10÷100, where X is the number of plankters. While only the larger plankters are counted in the “survey count” method, all the plankters are counted in the “total count” method.
1.3 Qualitative analysis of plankters:
The “differential count” method is usually followed which requires enumeration of some or all kinds of plankters, distinguishing them qualitatively into species or genera of phytoplankton and zooplankton. Shake well the diluted plankton sample and take 1 ml of plankton suspension in Sedgewick-Rafter counting cell or one drop on a glass slide and cover with cover slip and count following the method described for numerical count. Instead of counting the total number of plankton, count important groups of phytoplankton and zooplankton separately. Important groups of phytoplankton usually encountered are green algae (chlorophyceae), diatoms (Bacillariophyceae), blue-green algae (Cydnophyceae), dinoflagellates (Dinophyceae) and chrysomonads (Chrysophyceae). Zooplankton in ponds mainly comprise protozoans, rotifers, cladocerans, calanoid and cyclopoid copepods and their larval forms and occasionally nematodes and ostracods.
Based upon the total counts, percentage composition of the different forms as well as phytoplankton and zooplankton as a whole may be calculated with their seasonal variations.
2. Analysis of Benth Fauna
2.1 Sample collection:
Randomly fix sampling points covering various zones of the pond.
Collect sediment samples with the help of Ekman dredge for deeper ponds while glass tubes (both sides open; 7–10 cm dia and 30–40 cm long) for seasonal and shallow ponds. In case of sediment sample collection with tubes, the tube is gently placed on the sediment and then pushed further deep. The open end is then tightly closed with a rubber stopper and the tube is lifted up with the contents. The contents are emptied onto an enamel tray.
Transfer each sample into a separate tray.
Dilute the sample with pond water, stir the sediment gently and pass it through seive. BSS 40 (mesh size 0.4 mm for macrozoobenthos) or BSS 60 (mesh size 0.3 mm for meizoobenthos). Repeat the process till the samples are completely washed.
Transfer the sieved material to wide mouth bottles with little water in each and fix with 10% formaldehyde or 70% ethanol.
2.2 Quantitative analysis:
Numerical method:
Transfer the preserved samples into petridishes.
Segregate the organism into taxonomic groups with the help of pipette/forceps and magnifying glass or stereoscopic microscope.
Count them as total or under various taxonomic groupings and calculate the abundance of the organisms per unit area as per the following equation.
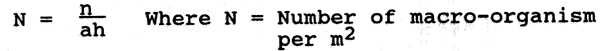
n = Number of macroorganism per sampled area
a = Area of Ekman dredge or area of tube sampler
h = Number of hauls constituting a sample
Volumetric Method:
Blot dry the sample organisms with the help of filter paper and segregate them into taxonomic groupings.
Transfer them to tubes calibrated at 1 ml intervals and add water from a burette drop by drop till the organisme is fully submerged in the water. Substract the amount of water added from the burette, from the test tube reading which will give the volume of benthic organism.
Compute the volume of benthic macro-organism per m2 as a whole or individual groupwise with the help of the following formula.

v = volume of macro-organisms/ sample
a = area of the Ekman's dredge/ area of the glass tube sampler
h = number of hauls constituting a sample.
Gravimetric Method:
Blot dry the samples group-wise on filter paper
Weigh them in a sensitive balance (wet weight)
Dry the above samples in an oven at 60–80°C to get dry weight (Exclude the shell weight of the molluscs)
The wet weight and dry weight of the benthos are expressed in g/m2.
APPENDIX V
| Case No.: | Date: | ||||
| Locality: | |||||
| Salient features of the water body: | |||||
| Length 1 mm: | Weight(g): | ||||
| Condition: | Fresh | Refrigerated | Frozen | Fixed | |
| Spoiled | |||||
| External examination: | ||||||||
| Look: | Emaciated | Healthy | ||||||
| Colouration: | Normal | Deeply pigmented | ||||||
| Other if any | … | |||||||
| Check for cysts, | spots, | lesions, | haemorrhages | |||||
| parasite | and abnormality if any | |||||||
| Body: | ||||||||
| Fins: | ||||||||
| Scales: | ||||||||
| Operculum: | ||||||||
| Eyes: | ||||||||
| Mouth cavity: | ||||||||
| Gills: | ||||||||
| Microscopic examination: | ||||||||
| Check for cysts, | parasite, | bacteria | ||||||
| Spores, | lesions, | inflamation | ||||||
| abnormality, | etc. | |||||||
| Mucous/Scales | ||||||||
| Fins | ||||||||
| Gills | ||||||||
| Liver | ||||||||
| Kidney | ||||||||
| Spleen | ||||||||
| Intestine | ||||||||
| Muscles | ||||||||
| Eye | ||||||||
APPENDIX VI
| DISEASE | CAUSATIVE AGENT | COMMON SYMPTOMS | TREATMENT MEASURES | |
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| A. | Bacterial diseases: | |||
| 1. | Columnaris disease | Flexibacter columnaris | Discoloured patches on the body, sloughing off of scales, erosion of gill lamellae, etc. | Copper sulphate 1 minute dip in 500 ppm solution 0.25–2 ppm in pond treat ment depending upon hardness of water. Hard water requires more. |
| Potassium permanganate 1 minute dip in 500 ppm solution; 3–5 ppm in pond treatment depending upon organic content. Organic rich water requires more. | ||||
| Penicillin + Streptomycin Injection for brood stock at 30–40 mg of streptomycin and 20 000 i.u. of penicillin/ kg body weight prevents stress mediated outbreaks. | ||||
| Terramycin (oxytetracycline) orally with feed at 7.5 g/100 kg/ day for 10–12 days. | ||||
| 2. | Bacteremia (Haemorrhagic septicaemia) | Aeromonas hydrophila, Pseudomonas fluorescens and possibly others | Shallow ulcerations, haemorrages and in severe cases the abdomen is swollen and the scales protrude. Internally the body cavity is filled with opaque fluid, paling of liver and sometimes heamorrhages over swim bladder. | Overcrowding, warmer conditions and oxygen depletion are some of the contributing conditions to be avoided. Terramycin (oxytetracycline) with feed at 7.5 g/100 kg body weight/day for 10–12 days. Furaxolidone at 5–7.5 g/100 kg body weight/day for 2–3 weeks. Pond treatment at 3–5 ppm of potassium permanganate is also a practical approach. |
| B. | Fungal diseases | |||
| 1. | Saprolegniosis | Saprolegnia spp. | Ulceration or exfoliation of the skin, fin erosion, exposure of muscles and jaw bones and in some cases tufts of minute white hair like outgrowths may occur in the affected regions. | Dip in treatment of 3% common salt solution or in 500 ppm copper sulphate solution or in 500–1 000 ppm of potassium permanganate solution till the first sign of any distress. Swabbing with 10 000 ppm of potassium dichromate is also recommended. |
| 2. | Branchiomycosis | Branchiomyces spp | Characterized by necrosis in the gill due to intravascular growth of this fungus. Histologically hyperplasia, fusing of gill lamellae and areas of acute necrosis are seen. | Improvement in water quality, avoidance of over feeding, manuring, decreasing organic level in the pond, addition of freshwater together with treatment measures suggested above are quite effective. Draining and liming the pond or treatment with bleaching powder is essential before initiating the next culture operation. |
| C. | Parasitic diseases: | |||
| 1. | Protozoan diseases | |||
| 1.1 | Ichthyophthiriasis (white spot disease) | Ichthyophthirius sp. | Presence of pin point size numerous white spots on the body, fins, gills, etc. The parasite can be observed in skin smear by its round ciliated body and horseshoe shaped nucleus. The disease is common in nursery and rearring pond causing large scale mortality. | Mixture of malachite green and formalin at several concentrations are very effective. 0.05 ppm of malachite green and 25–50 ppm of formalin can be used as prolonged bath. Spraying the entire pond area with malachite green at 0.15 ppm is very effective provided that 3 such applications are made at 3 days intervals. Application of quick lime (CaO) at higher rate in the pond is also very effective. Several other antiprotozoan drugs are also in use against this disease. |
| 1.2 | Trichodinosis | Trichodina sp. | Discolouration of the body, presence of thick mucous coat on the affected surface, frayed fins and gills are some of the common characteristics. Smear from gills and skin readily exhibits parasites with radial ciliary band and central denticles. | Bathing in 1–2% solution of sodium chloride, 150–250 ppm of formalin, 0.25 ppm of malachite green are very effective measures. Affected ponds should be disinfected before next stocking. |
| 1.3 | Myxosporodiosis | Myxosporidian sp. | Presence of white cysts of varying diameters on the body, fins, gills, opercula, etc. In some cases, emaciation, dark colouration together with presence of cysts and spores in kidney tissues without showing external cysts. | Infected fish should be immediately removed from the pond. Before inititing the next culture operation the pond should be dried if possible and/or thoroughly disinfected with bleaching powder at 50 ppm. Provision of settling tank before the water intake in the pond also reduces the risk of infection. |
| 2. | Metazoan disease | |||
| 2.1 | Monogenetic trematode infection | Gyrodactylus sp. and Dactylogyrus sp. | Heavily infected fish show increased production of mucous, frayed fins, skin ulcers and damaged gills. Microscopic observation of the skin lesion/smear and temporary mount of a portion of gill show the presence of the parasites. | Bath in 100–250 ppm of formalin ranging from 1 to 3 hours, is very effective. Dip in 2–5% salt solution till the first sign of distress is equally beneficial. Bath or pond treatment with some soft organophosphorus insecticide is also equally effective. |
| 2.2 | Black spot disease | Diplostomum sp. | Development of small black or brown spots on several parts of the body and fins. Specific locations are cutis and under lying muscles. Microscopic examination and dissection helps in locating rolled up and slowly moving worms embedded in the connective tissue. | Fish exhibiting black spots may be given an hour bath in 10 ppm picric acid solution. Removal of aquatic snails and preventing the entry of birds are some of the preventive measures. Infection does not spread from fish to fish and hence it is not worth treating uninfected stock. |
| 2.3 | Argulosis | Argulus sp. | Development of haemorrhagic patches over the body and presence of the parasite in large number in and around the lesion. | Benzene hexachloride application in pond at 0.02 ppm a second subsequent treatment after a week. Affected fish should also be given dip in 500–1 000 ppm potassium permanganate solution which helps in avoiding secondary infection as well as accelerate the healing process. Malathion at 0.25 ppm in pond also effectively controls the infection. Malathion also required a second treatment after a week interval. |
| 2.4 | Leraeasis | Lernaea sp. | Anaemia, severe ulcerations and presence of attached cylindrical parasite of 1 to 2 cm length hanging outside. Sometimes cause mass mortality in carp nursery and rearing ponds. | Baths in concentrated solution of salt and potassium permanganate is reported to be effective. However, the author has found very little improvement by potassium permanganate treatment. Juveniles are embeded in the skin and hence remain unaffected. Chlorophos a Diptrex or Neguvan when applied in the pond at 0.25 ppm kills all the parasite. Bromex completely cures the infection when applied at 0.15 ppm. |
| 2.5 | Leech infection | Piscicola sp. | Relatively they are not dangerous. They affect the fish by their attachment and feeding. Area of attachment normally exhibit excessive mucous production, hyperaemia and petechial haemorrages. Inflamation and epithelial hyperplasia extending through the dermis may be observed. Open wounds are often infected by bacteria and fungi. Attacked fish show attached parasite, irritation and restlesness. They may attempt to rub against objects. | Removal of aquatic vegetation and maintenance of pond hygene is the most important preventive measure. Hard objects such stones, logs, etc. should also be removed. Disinfection of pond with unslaked lime at 2 500–3 000 kg/ha should be done prior to next rearing operation. Short bath in 3–5% salt solution is also very effective treatment. Dip in 1 000 ppm acetic acid or 10 000 ppm in potassium permanganate solution are also quite effective measures. Organophosphorus insecticides as described in earlier cases can also be used. |
APPENDIX VII
Book Keeping
Book keeping is the core of fish farm management which records all aspects of fish farm operations and enable the fish farmer/farm manager or the extension officer to understand the economics of the pond/farm operation, provide information for planning developmental projects and better services for fish farmers, and also to provide necessary ground to get funding support from financial institutions.
The book-keeping system has the following two major aspects of recording:
Account keeping
Operational activities
A simplified form of this system is described which can be used by fish farmers/fish farm managers and extension workers.
A. Account Keeping
Maintain 2 thick bound registers one as Cash Book (CB) and the other as Ledger Book (LB). Number the pages in Cash Book keeping the same page number for both right and left facing pages. Keep left pages for receipts and right pages for payments. Calculate closing balance (CB) for the day which will become opening balance (OB) for the following day. Number all pages of the Ledger Book (LB) and keep at least one page for each item as shown by giving examples of 8 pages. Number of pages for each item depends on the extent of recurring expenditures or receipts under that head. Accordingly, enough page space should be kept under that head so that it may cover a period of 1 year. Enter the details of receipts and payments on daily basis in both of these registers. To analyse the performance of individual sector or a particular pond of the farm, pond-wise or sectorwise separate entries should be made in the ledger book and in such cases separate pages should also be provided for each pond. For example, for fish sale, there should be separate pages for each pond. Accordingly, entries should be made under fish sale of pond No. 1 or Fish sale of pond No. 2, etc. This will give a complete record of everything you spend and any money you receive.
1. Cash Book (CB)
Receipts always on left page of the Cash Book
| Receipts | Page 1/L | ||||
|---|---|---|---|---|---|
| Date | Particulars of receipts | Ledger Book Page No. | Amount (US$) | ||
| 7.1.1987 | Capital Acct. | ||||
| Received loan money from the State Bank of India, Bhubaneswar | 1 | 2 500.00 | |||
| 2 500.00 | |||||
| 8.1.1987 | Opening balance (OB) | 1 000.00 | |||
| Sale proceeds (fish) | |||||
| Received towards sale of 100 kg of unwanted fish at US$ 1.50/kg after bleaching powder application | 2 | 150.00 | |||
| Sale proceeds fingerlings | |||||
| Received towards sale of 50 000 fingerlings of catla at US$ 200/1 000 | 3 | 10 000.00 | |||
| 11 150.00 | |||||
| 9.1.1987 | Opening balance | 2 106.00 | |||
Payments always on right page of the Cash Book
| Payments | Page 1/R | ||||
|---|---|---|---|---|---|
| Date | Particulars of expenditure | Ledger Book page No. | Amount (US$) | ||
| 7.1.1987 | Pond Construction | ||||
| Construction of one nursery pond | 4 | 1 000.00 | |||
| Maintenance of Pond | |||||
| Repair of dyke of stocking pond No.7 | 5 | 500.00 | |||
| Total: | 1 500.00 | ||||
| Closing balance | 1 000.00 | ||||
| 2 500.00 | |||||
| 8.1.1987 | Labour Charge | ||||
| 2 labourers for pond poisoning at US$ 20.00 per labourer/day | 6 | 40.00 | |||
| Piscicide | |||||
| 100 kg of bleaching powder at US$ 4.00/kg | 7 | 400.00 | |||
| Total: | 440.00 | ||||
| Closing balance | 2 106.00 | ||||
| 2 506.00 | |||||
2. Ledger Book (LB)
| Capital | Page 1 | |||
|---|---|---|---|---|
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 7.1.87 | Loan money from State Bank of India, Bhubaneswar Branch | 1 | 2 500.00 | |
| Fish Sale | Page 2 | |||
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 8.1.87 | 100 kg of unwanted fish sold at US$ 1.50/kg | 1 | 150.00 | |
Ledger Book (LB)
| Fry Sale | Page 3 | |||
|---|---|---|---|---|
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 8.1.87 | Sale of 50 000 catla fingerlings at 200/1 000 US$ | 1 | 10 000.00 | |
| Pond construction | Page 4 | |||
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 7.1.87 | Construction of one nursery pond | 1 | 1 000.00 | |
Ledger Book (LB)
| Maintenance of pond | Page 5 | |||
|---|---|---|---|---|
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 7.1.87 | Repair of pond dyke of stocking pond No. 7 | 1 | 500.00 | |
| Maintenance of pond | Page 6 | |||
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 8.1.87 | 2 labourers for application of bleaching powder for pond poisoning at US$ 20.00 per labourer | 1 | 40.00 | |
Ledger Book (LB)
| Piscicide | Page 7 | |||
|---|---|---|---|---|
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| 8.1.87 | 100 kg of bleaching powder e US$ 4.00/kg | 1 | 400.00 | |
| Fish Feed | Page 8 | |||
| Date | Particulars | CB page No. | Debit Amount (US$) | Credit Amount (US$) |
| Item | Present | Expected | Annual | Monthly |
3. Annual Balance Sheet
An Annual Balance Sheet form should be prepared after a year of farm/pond operation which will show how much is earned and what the fish farm is worth. It makes a summary of everything that has been recorded in cash book (CB) and ledger book (LB). Make total of every item in the LB and put it in the Annual Balance Sheet. If required Monthly Balance Sheet can also be prepared taking monthly total of every item, from the LB.
4. Depreciation Cost
Depreciation cost is the amount of value an expensive item loses every year and this amount one must keep aside to replace the item when it is worn out. To work out depreciation cost for any item, for example a pump set, one should consider the following two aspects:
What would be its expected life? Say 10 years
What is the present value?
For each such item put these two figures in respective column in the following form and calculate annual or monthly depreciation cost.
| Item (Asset) | Present cost | Expected life | Annual depreciation | Monthly depreciation |
|---|---|---|---|---|
| Pump set | $ 1 000 | 10 years | $ 100 | $ 8.33 |
| Net | $ 500 | 6 years | $ 83.3 | $ 6.94 |
1.3 Annual Balance Sheet
YEAR …
| Month | Cost of Production | Sales Income | Net Income* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pond/farm construction | Maintainance of pond/farm | Labour charges | Piscicide | Fish seed | Fish feed | Spares etc. | Total Fish | Fish seed | Total | ||
| January | |||||||||||
| February | |||||||||||
| March | |||||||||||
| April | |||||||||||
| May | |||||||||||
| June | |||||||||||
| July | |||||||||||
| August | |||||||||||
| September | |||||||||||
| October | |||||||||||
| November | |||||||||||
| December | |||||||||||
* Net Income = Sales Income - Cost of Production
5. Loan Accounting
A separate loan record sheet should also be maintained if the farmer has taken any loan for fish farming. For example, if the farmer has taken a loan of $ 500 from the Government for pond construction and that has to be repaid in 10 years with an annual interest rate of 10%, with the assistance of the Extension Officer, the fish farmer should keep a record of his loan repayment. The interest paid on the loan should be regarded as a production cost and should be taken into consideration in calculating the net income of the fish farming operation. A simple loan record sheet is given below:
| Loan 1 | Loan 2 | ||
|---|---|---|---|
| Source: | State Bank of India Muzaffarpur | Source: | Bank of India, Muzaffarpur |
| Date … | Date… | ||
| Amount… | Amount… | ||
| Period of repayment… | Period of repayment… | ||
| Annual rate of interest… | Annual rate of interest… | ||
| Repayment Detail | Repayment Detail | ||||||
|---|---|---|---|---|---|---|---|
| Date | Interest Payment | Loan Repayment | Loan Outstanding | Date | Interest Payment | Loan Repayment | Loan Outstanding |
| Total: | |||||||
B. Operational Activities
Aspects pertaining to the description of the ponds/farm, plan of work, operational activities such as prestocking, stocking and poststocking operations, monthly sampling details, harvesting, induced breeding, fish seed rearing, etc. should also be recorded. Formats, with examples, for recording such activities are presented hereunder.
1. Pond Description
| POND NO. DESCRIPTION | 1 | 2 | 3 |
| Age(years | 5 | 4 | 1/2 |
| Nature of earlier operations if any | Composite fish culture | Culture of 3 Indian major carps | Rearing of fish seed |
| Average annual Production rate | 5 500 kg/ha | 3 400 kg/ha | |
| Pond area (m2) | 2 000 | 3 000 | 200 |
| Water depth (m) | 2.5 | 1.5 | 0.7 |
| Sediment depth (cm) | 15 | 10 | 8 |
| Water source | Irrigation canal | Rain | Rain |
2. Farming plan:
| POND NO. FARMING PLAN | 1 | 2 |
| Type of farming | Composite fish culture | Fish seed rearing |
| Species | Catla, rohu, mrigal, silver carp, grass carp, common carp | Silver carp, grass carp, |
| Stocking density | 6 000/ha | 6 million/ha |
| Manuring | Cowdung | Poultry manure |
| Feeding | Fish feed | Micro-encapsulated feed |
| Period of rearing | 12 months | 3 weeks |
3. Pre-stocking operations:
| POND NO. OPERATIONS | 1 | 2 |
|---|---|---|
| 1. Pond clearing | Weed clearing using manual method | Weed clearing using weedicides |
| 2. Eradication of unwanted fish | Poisoning-250 kg of bleaching powder applied in the pond at 50 ppm | Poisoning-1 125 kg of mahua oil cake at 250 ppm |
| 3. Liming | 60 kg of lime at 200 kg/ha | |
| 4. Organic manuring | ||
4. Stocking details:
| POND NO. STOCKING DETAILS | 1 | 2 |
|---|---|---|
| 1. Date of stocking | 15.11.86 | 10.10.86 |
| 2. Species stocked | Catla (C), rohu (R), Mrigal (M), Silver carp (S) , Grass carp (G), Common carp (CC) | R, C, R, M |
| 3. Stocking density | 6 000/ha | 5 000/ha |
| 4. Number stocked | 1 200 | 1 500 |
| 5. Species ratio | C 2; R 3; M 1.5; S 1.5; G 0.5; CC 1.5 | C 4; R 3; M 3 |
| 6. Seed treatment | Short bath in potas- sium permanganate solution | Short bath in 2% salt solution |
| 7. Average weight (g) | C-50; R-60; M-50; S-40; G-50; CC-30 | C-50; R-60; M-50 |
5. Post-stocking operations:
POND NO.
| MONTH OPERATIONS | November | December | October |
|---|---|---|---|
| Organic manuring | |||
| Liming | |||
| Inorganic fertilizer | |||
| Feeding | |||
| Medication | |||
6. Monthly sampling details (growth):

| DATE SPECIES | 1.12.86 A/B | 2.1.87 A/B |
|---|---|---|
| Catla | 50/10 | 70/20 |
| Rohu | 40/8 | 60/20 |
| Mrigal | 30/8 | 48/18 |
| Silver carp | 35/15 | 55/20 |
| Grass carp | 42/16 | 60/18 |
| Common carp | 48/18 | 65/17 |
7. Standing crop of fish (estimation)
POND NO. 1 MONTH: DECEMBER 1986
| Species | Av. Wt. attained (g) | No. stocked | Mortality | Total weight (kg) |
|---|---|---|---|---|
| Catla | 150 | 240 | 10 | 34.5 |
| Rohu | 140 | 360 | - | 50.4 |
| Mrigal | 130 | 180 | - | 23.4 |
| Silver carp | 185 | 180 | - | 33.3 |
| Grass carp | 190 | 60 | - | 11.4 |
| Common carp | 90 | 180 | 10 | 15.3 |
| Total | 1 200 | 20 | 168.3 | |
8. Harvesting details (Table size fish production):
POND NO. POND SIZE: DATE:
| SPECIES DETAILS | Catla | Rohu | Mrigal | Silver carp | Grass carp | Common carp | Total |
|---|---|---|---|---|---|---|---|
| No. stocked | |||||||
| No. harvested | |||||||
| Survival (%) | |||||||
| Average | |||||||
| weight (g) | |||||||
| Total | |||||||
| weight (kg) | |||||||
| Species | |||||||
| Contribution % | |||||||
Gross Production (Kg) = Total weight of harvest (Kg)
Net Production (Kg) = Gross production (Kg) - Initial stocking weight (Kg)
9. Harvesting details (Fish seed rearing):
| POND NO. | Period of rearing (days) | Species | No. harvested | Survival % | Av. Wt. (g) | Remarks |
|---|---|---|---|---|---|---|
10. Induced breeding:
| DATE | Species | Weight (g) | Inducing agent Dose (mg/kg) | Sets attempted (No.) | Spawning success (%) | Estimated No. of spawn | Remarks | |
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
APPENDIX VIII
Essential Items for a Farm (Self Sufficient 5 ha Unit)
A. Table size fish farming sector:
| 1. | Nylon seive net pieces with head rope, foot rope, sinkers and floats. | |
| 40 mm meshed (10 m × 6 m) - 10 pieces | ||
| 20 mm meshed (10 m × 6 m) - 10 pieces | ||
| (Mesh size should be measured knot to knot diagonally) | ||
| 2. | Hand net (Scoop net) of 25 mm mesh | |
| Nylon material with aluminium/cane framing | ||
| open at both ends - 5 | ||
| closed at distal end - 5 | ||
| 3. | Spring balances of the following capacities: | |
| 1 kg — 2 | ||
| Dial type 5 kg — 2 | ||
| Dial type 20 kg — 2 | ||
| 4. | Plastic buckets with lids of the following capacities: | |
| 2 l – 6 | ||
| 5 l – 6 | ||
| 12 l – 6 | ||
| 25 l – 2 | ||
| 5. | Plastic or enamel trays - 6 | |
| 6. | Plastic tub/galvanized iron sheet tub/ fibreglass circular tank- 5 | |
| 7. | Spade | - 5 |
| 8. | Bottom raker | - 2 |
| 9. | Sickle | - 6 |
| 10. | Pick-axe | - 2 |
| 11. | Grass cutting knives | - 6 |
| 12. | Crowbar | - 4 |
| 13. | Hammer | - 2 |
| 14. | Rope of various sizes | - 1 roll each |
| 15. | Torches (3 celled) | - 4 |
| 16. | 5 HP diesel pump set with generator set attachment option | - 1 |
| 17. | Generator set to be driven by 5 HP diesel engine | - 1 |
| 18. | Small boat | - 1 |
| 19. | Mini tractor with trailer | - 1 |
| 20. | Bamboo hanger for drying | |
| the net | - 1 | |
| 21. | Cane baskets | - 10 |
| 22. | Anti-poaching devices: | |
| - Unfinished bamboo | - 100 | |
| - Bamboo poles | - 200 | |
| - Barbed wire | - 5 rolls | |
| 23. | Nylon twine (assorted size) | - 1 kg |
| 24. | Conditioning hapa (cotton) | - 10 |
| 25. | Towels | - 4 |
| 26. | Bamboo baskets (50 kg capacity) | - 10 |
| 27. | 200 kg capacity balance with tripod stand and set of weights | - 1 |
| 28. | Spare gunny bags | - 20 |
| 29. | Umbrella | - 4 |
| 30. | Rain coat | - 6 |
| 31. | Gum boot | - 6 pairs |
| 32. | Fish measuring board | - 2 |
| 33. | Feeding tray (galvanized iron sheet) (50 cm × 100 cm × 15 cm) | - 20 |
| 34. | Mini tractor operated compressor | - 1 |
B. Fish Seed Production Sector:
In addition to the items listed under A, the following items are also needed.
| 1. | Nylon seive net pieces complete with head rope, foot rope, sinkers and floats | |
| 1.5 mm meshed (10 m × 5 m ) | - 2 | |
| 3 mm – 4 mm meshed (10 m × 5 m) | - 5 | |
| 2. | Hand net (scoop net) with opening at both ends and having a thick twine at the distal end for tying. | |
| 25 mm meshed nylon netting | - 12 | |
| 3 mm – 4 mm meshed nylon/cotton netting | - 5 | |
| 3. | Canvas strechers with provision of net cover for brood fish transport in the farm | - 2 |
| 4. | Nylon breeding hapa | - 10 |
| 5. | Nylon hatching hapa: Outer | - 100 |
| Inner | - 50 | |
| 6. | Bamboo poles | - 150 |
| 7. | Jute or cotton twine | - 2 kg |
| 8. | Cheesecloth for holding brood fish | - 5 m |
| 9. | Plastic buckets graduated | |
| 1 l | - 10 | |
| 5 l | - 10 | |
| 12 l | - 10 | |
| 10. | Plastic/enamel mug graduated | - 6 |
| 11. | Enamel tray | - 10 |
| 12. | Enamel basins 3–5 1 capacity | - 10 |
| 13. | Feathers | - 50 |
| 14. | Folding work table | - 1 |
| 15. | Folding chairs | - 4 |
| 16. | Set of dissection instruments | - 2 |
| 17. | Centrifuge machine (hand operated) | - 1 |
| 18. | Centrifuge tubes graduated | - 20 |
| 19. | Petridishes (assortment) | - 20 |
| 20. | Dropper with long nozzle | - 20 |
| 21. | Tissue homogenizer | - 5 |
| 22. | Beaker - 50 ml capacity | - 10 |
| 100 ml capacity | - 10 | |
| 250 ml capacity | - 10 | |
| 23. | Clean homoeopathic tube with stopper | - 200 |
| 24. | Hypodermic syringes - 2 cc capacity | - 5 |
| 5 cc capacity | - 5 | |
| 25. | Hypodermic needles | |
| No. 20 | - 12 | |
| No. 21 | - 12 | |
| No. 22 | - 12 | |
| 26. | Pituitary gland | - 1 000 nos. (10 000 mg) |
| 27. | Absolute alcohol | - 450 ml × 2 |
| 28. | Distilled water (sterile) | - 200 ampoules |
| 29. | Spawn measuring cup 10, 25, 50 ml capacity | - 1 each |
| 30. | Strainer cup for measuring fry 100 ml, 500 ml | - 2 each |
| 31. | Oxygen cylinder with regulator pressure gauge and dry oxygen gas | - 2 |
| 32. | Plastic bags/cylindrical rolls thickness 0.3 - 0.5 mm circumference 100–150 mm | - 10 rolls/5 000 nos. |
| 33. | Thermometer (0–50°C) | - 2 |
| 34. | Brushes for cleaning metal, plastic, glass appliances (assorted sizes) | - 20 |
| 35. | Stereoscopic microscope with stage lightning | - 1 |
| 36. | High power hand lense | - 1 |
| 37. | Cotton twine for tying the oxygen packed bags | - 5 kg |
| 38. | Butcher's knife | - 2 |
| 39. | Acetone | - 450 ml × 2 |
| 40. | Desicator with silica gel | - 2 |
| 41. | Porcelain pestle and mortar (5–10 cm dia) | - 2 |
| 42. | Widemouth bottle with glass stoppers | - 10 |
| 43. | Cotton wool | - 2 kg |
| 44. | Rubber cushion (60 cm × 40 cm × 5 mm) | - 2 |
| 45. | Cathetors (2.5 mm dia) | - 2 |
C. Piscicides, feeds, manures and fertilizers
Bleaching powder/mahua oil cake
Rice polish
Groundnut/mustard oil/soyabean cake
Mineral mixture
Fish meal
Raw cow dung/poultry manure/pig dung
Urea
Ammonium sulphate
Super phosphate
Muriate of potash
Lime
To avoid storage loss of nutrients and spoilage, it is desirable to buy the items on regular basis. The selection of items also depends upon the local availability and relative market prices. However, the store should have sufficient amount of ready stock of these items so that they may last for 3–4 weeks.
C. Medicine Chest
| Sodium choride (common salt) | - 5 kg |
| Copper sulphate | - 5 kg |
| Potassium permanganate | - 500 g × 10 |
| Organophosphate insecticide (Malathion/Somithion) | - 1 l × 5 |
| Benzenehexachloride (BHC) wettable powder | - 500 g × 10 packs |
| Formaldehyde (formalin) | - 10 l × 1 |
| Acetic acid (glacial) | - 500 ml × 10 |
| Quick lime | - 50 kg × 5 packs |
| Bleaching powder (sodium hupochlorite) | - 25 kg × 10 bags |
| Oxytetracycline | - 100 g × 10 |
| Penicillin | - 10 vials |
| Streptomycin | - 10 vials |
| Malachite green (zinc free salt) | - 10 g × 5 |
