3.1. Description of the tanning-process
3.2. Emissions
3.3. Prevention of waste production
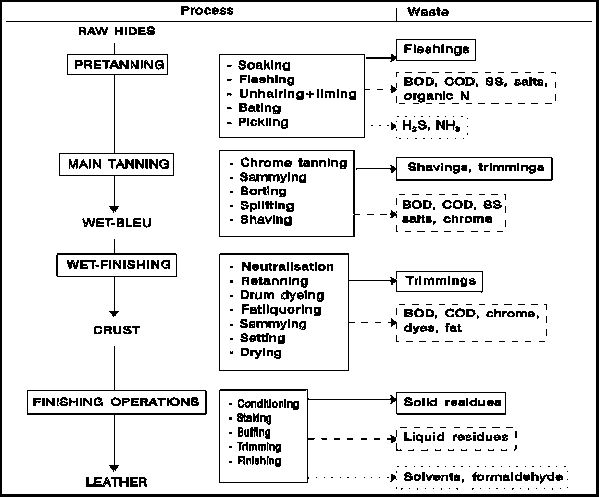
Figure 3 presents a flow diagram of a the tanning-process. Hides are a by-product of slaughter activities and can be processed into a wide range of end products. For each end product, the tanning process is different and the kind and amount of waste produced may vary enormously.
The chemicals traditionally used for tanning have been derived from plants, whereas the most common process nowadays is a combination of chrome salts (chrome tanning) and readily usable vegetable extracts (vegetable tanning) (Buljan 1994). While chrome tanned shoe leather is the most widely produced leather, this kind of leather will receive most attention in the following.
In most cases raw hides produced at slaughterhouses are preserved by pickling and drying for transport to tanneries and further treatment. In the very few cases that hides are instantly tanned there is no need for preservation. During the tanning process at least ±300 kg chemicals (lime, salt etc.) is added per ton of hides.
Pretanning (Beamhouse operations)
Soaking:
The preserved raw hides regain their normal water contents. Dirt, manure, blood, preservatives (sodiumchloride, bactericides) etc. are removed.
Fleshing and trimming:
Extraneous tissue is removed. Unhairing is done by chemical dissolution of the hair and epidermis with an alkaline medium of sulphide and lime. When after skinning at the slaughterhouse, the hide appears to contain excessive meat, fleshing usually precedes unhairing and liming.
Bating:
The unhaired, fleshed and alkaline hides are neutralised (deliming) with acid ammonium salts and treated with enzymes, similar to those found in the digestive system, to remove hair remnants and to degrade proteins. During this process hair roots and pigments are removed. The hides become somewhat softer by this enzyme treatment.
Pickling:
Pickling increases the acidity of the hide to a pH of 3, enabling chromium tannins to enter the hide. Salts are added to prevent the hide from swelling. For preservation purposes, 0.03 - 2 weight percent of fungicides and bactericides are applied.
Tanning
There are two possible processes:
1: Chrome tanning:After pickling, when the pH is low, chromium salts (Cr3+) are added. To fixate the chromium, the pH is slowly increased through addition of a base. The process of chromium tanning is based on the cross-linkage of chromium ions with free carboxyl groups in the collagen. It makes the hide resistant to bacteria and high temperature. The chromium-tanned hide contains about 2-3 dry weight percent of Cr3+. Wetblue, i.e. the raw hide after the chrome-tanning process, has about 40 percent of dry matter.2: Vegetable tanning:
Vegetable tanning is usually accomplished in a series of vats (first the rocker-section vats in which the liquor is agitated and second the lay-away vats without agitation) with increasing concentrations of tanning liquor. Vegetable tannins are polyphenolic compounds of two types: hydrolysable tannins (i.e. chestnut and myrobalan) which are derivatives of pyrogallols and condensed tannins (i.e. hemlock and wattle) which are derivatives from catechol. Vegetable tanning probably results from hydrogen bonding of the tanning phenolic groups to the peptide bonds of the protein chains. In some cases as much as 50% by weight of tannin is incorporated into the hide (Ockermann and Hansen, 1988).
Finishing
Wetblue:
Chromium tanned hides are often retanned - during which process the desirable properties of more than one tanning agent are combined - and treated with dye and fat to obtain the proper filling, smoothness and colour. Before actual drying is allowed to take place, the surplus water is removed to make the hides suitable for splitting and shaving. Splitting and shaving is done to obtain the desired thickness of the hide. The most common way of drying is vacuum drying. Cooling water used in this process is usually circulated and is not contaminated.
Crust:
The crust that results after retanning and drying, is subjected to a number of finishing operations. The purpose of these operations is to make the hide softer and to mask small mistakes. The hide is treated with an organic solvent or water based dye and varnish. The finished end product has between 66 and 85 weight percent of dry matter.
A more detailed description of the tanning process is found in the publication “Animal by-product processing” by Ockerman and Hansen, 1988.
3.2.1. Solid waste
3.2.2. Wastewater
3.2.3. Air pollution
The discharge of solid waste and wastewater containing chromium is the main environmental problem. Chromium is a highly toxic compound and the dumping of chromium containing material is in most countries restricted to a few special dumping grounds. Reduction of chromium discharge is therefore essential. Emissions into the air are primarily related to energy use, but also the use of organic solvents and dyes causes emissions into the air.
The production of fresh hides has been estimated at about 8-9 million tonnes per year (FAO, 1990a). During the processing of these hides a total of 1.4 million tonnes of solid waste is produced (El Boushy and Van der Poel, 1994). This means that in all likelihood ca 16% of the processed hides is leather waste. Buljan (1994) puts the figures for trimmings and splittings (i.e. leather waste) at a total of 225 kg/ton hide (i.e. ca 23%). This is almost the same amount of waste produced as meat from fleshing activities (7 - 23%). For every ton of raw hide processed, the amounts of solid waste and by-products may be produced as given in Table 14 (Buljan, 1994). These figures show that the solid waste produced per ton of raw hide is about 450-600 kg. About half of this contains 3% chrome on a dry matter basis.
|
|
Pretanning |
Tanning |
Finishing |
|
Trimmings |
120* |
110 |
32 |
|
Fleshings |
70-230 |
|
|
|
Wet blue split |
|
115 |
|
|
Buffing dust |
|
|
2 |
|
Total |
190-350 |
225 |
34 |
|
GRAND TOTAL |
Approx. 450-600 |
||
*: hides not trimmed in the abattoir itself
Buljan (1994) states:
“Collection and safe disposal of solid waste, especially chrome containing solid waste and sludge is normally monitored by environmental authorities and associated with costs. Conversion of solid waste into by-products not only reduce pollution load, it can also be commercially beneficial. This represents great potential for producing increased returns to tannery processing through deriving value from wastes. In any event, reduction of waste is essential in order to meet demands for reduced pollution load from tanneries.”
As for the production of wastewater, over 80 per cent of the organic pollution load in BOD terms emanates from the beamhouse (pretanning); much of this comes from degraded hide/skin and hair matter. The beamhouse is also the source of all non-limed and limed solid waste such as fleshing, trimming and waste split. As already mentioned, during the tanning process at least ca 300 kg of chemicals (lime, salt etc.) are added per ton of hides. Excess of non-used salts will appear in the wastewater. Because of the changing pH, these compounds can precipitate and contribute to the amount of solid waste or suspended solids (Department of the Environment, 1978).
Every tanning process step, with exception of the crust finishing operations, produces wastewater. An average of 35 m3 is produced per ton of raw hide. This wastewater contains:
- salts (Cl), fat, protein, preservatives (soaking);- lime and ammonium salts, ammonia, protein (hair), and sulphides (fleshing, trimming, bating);
- chromium(salts) and polyphenolic compounds (tanning); and
- dye and solvent chemicals (wet-finishing).
Solid waste produced consists of fleshings containing lime, chromium containing ‘wet-blue’ shavings and of trimmings (leather).
Water will not only have a diluting effect, it also increases the number of kg of BOD per ton of hides. Rajamani (1987) gives a BOD range of 1000 - 3000 mg/l depending upon the volume of water used and on other impurities. TNO gives BOD and COD values both for precipitated and mixed wastewater. BOD- and COD-values for precipitated wastewater show a reduction of BOD and COD of ca 50% (Pelckmans, undated). This implies that it is worth precipitating dissolved organic compounds and treating this as solid waste. It is known that treatment of solid waste can in general be undertaken without too many efforts and that the costs and energy required are lower than those for the treatment of wastewater.
Tanneries that perform the complete tanning procedure, produce mixed wastewater. The composition of this wastewater is not solely the result of separate waste streams that merge together. The different pH’s and the different compounds influence each others’ solubility. In composite wastewater, compounds precipitate while they stay dissolved in the wastewater from the separate processes (Pelckmans, undated). Most reports give reliable values for composite wastewater. Some reports only give data for the separate wastewater streams. These values should be used with great care and should not be merely added in order to arrive at a compound value.
In Table 15 high and low values for BOD, COD, SS and Cr3+ are given. This variation might be caused by a high amount (45 m3 per ton of hide) or low amount (25 m3 per ton of hide) of water used during the tanning process. Mulder and Buijssen (1994) give values of 50 m3 per ton of hide for traditional manufacturing processes of Wet-blue and 20 m3 per ton of hide when water saving actions are applied.
|
|
(1) |
(2) |
(3) |
|
BOD |
110 |
40-100 |
80 |
|
COD |
265 |
120-280 |
|
|
SS |
216 |
70-200 |
|
|
Cr |
8.8 |
5 |
|
(1): Rajamani (1987); values from Kanpur, Pakistan.
(2): Clonfero (1990); refering to a UNIDO-study (1975).
(3): Taiganides (1987); an average and quit general value.
In Table 16, RIVM (1992) presents the quantity and composition of wastewater for every step of the tanning process in a Dutch situation. Per ton of hide a total of 35 m3 wastewater is produced. The Dutch figures of the COD produced during the pretanning process are higher than the figures mentioned in Table 15. RIVM noted that measured chromium-concentrations were 3-7 times higher than the estimated figures. Moreover, in the Netherlands about 50% of the hides processed in tanneries have already been pretanned or tanned.
|
Process step |
Amount of water |
pH |
COD |
NKj |
Cr |
|
|
(m3/ton) |
- |
kg COD/m3 |
kg N/m3 |
kg Cr/m3 |
||
|
Pretanning: |
|
|
|
|
|
|
|
|
Soaking |
4-6 |
6-9 |
30-40 |
1-1.5 |
- |
|
|
Unhairing, liming |
5-9 |
12-13 |
40-60 |
3-5 |
- |
|
|
Fleshing |
1-3 |
- |
- |
- |
- |
|
|
Deliming, bating |
5-7 |
8.5-9 |
5-8 |
3.5-4 |
- |
|
Tanning: |
|
|
|
|
|
|
|
|
Chrome tanning |
0.5-1 |
3.8-4 |
2-3 |
0.3-0.6 |
0.5-5 |
|
|
Pressing |
0.4-0.6 |
3.6-4.5 |
1.2-1.8 |
0.11-0.22 |
0.5-5 |
|
|
Neutralisation |
1-1.5 |
4.5-4.7 |
2.5-3 |
0.5-0.8 |
0-1.0 |
|
|
Painting, fatting |
3-4.5 |
3.8-4.5 |
5-6 |
0.2-0.3 |
0-5.0 |
|
Finishing: |
|
|
|
|
|
|
|
|
Drying |
3-6 |
|
|
|
|
|
|
Finishing |
1-2 |
|
|
|
|
|
|
Cleaning |
5 |
|
|
|
|
Table 17 gives the emissions into the air during the tanning process. Few figures are available about the amount of air pollution.
An important part of the air pollution by leather tanneries is caused by the need for energy. RIVM (1992) estimated the need for the Dutch tanneries at: 439 kWh (electricity) per ton of raw hides and 108 m3 of gas per ton of raw hides. Gas is used for heating. Table 17 gives the emissions into the air as a result of gas-combustion. No figures are available about the emissions into the air as resulting from the use of electricity.
|
Process-step |
Air pollutants |
kg/ton raw hide |
|
Unhairing/liming |
H2S |
|
|
Deliming/Bating |
NH3 |
|
|
Finishing |
solvents, formaldehyde |
25* |
|
heating with gas |
CO |
0.033* |
|
CO2 |
190* |
|
|
NO2 |
0.17* |
*: Netherlands situation, based on figures of RIVM (1992)
H2S may be emitted into the air when the pH of the processwater is less then 7. During the finishing-process volatile organic compounds are used.
Considerations for the reduction of the amount of polluting value of the produced wastewater are:
- a reduction of the total water use by re-use of produced wastewater and by the development of technologies that minimize the quantity of water needed during the tanning process; and- a reduction of the used chemicals such as lime, salt, sulphide etc and a reduction of chromium.
The following gives a more detailed discussion (from Higham, 1991).
Water conservation
A reduction of water use can lead to a reduction of the total waste load. Re-use of wastewater with a minimal harmful or even a moderately beneficial effect on earlier processes may be considered as an option.
Curing hides and skins
A reduction of the use of salt for preservation can be considered as an option. Fifteen percent of salt on weight basis may preserve the hides for even 6 weeks, and 5 per cent of salt plus biocide lead to a preservation for two months. Chilling without salt can preserve hides for a few days. Another alternative preservation method is radiation by electron beam or gamma rays. Where possible, biodegradable preservatives (insecticides etc.) should be used instead of derivatives of chlorinated aromatic hydrocarbons. The latter persist in the waste and are highly toxic to the environment.
Beamhouse processes
Hair saving methods are recommended to prevent degraded keratin from entering the waste streams. Unhairing/liming fluids can be recycled after recharging. It is also recommended that the unhairing and liming stages should be seperated. Both liquids can be recharged and hair can be screened out. The intermediate wash can be re-used as a soak liquid.
Tanning
Low chrome systems, possibly requiring an aluminium salt for pretannage will produce a wet-white leather. Splitting and shaving wastes will contain less chromium. Alternative mineral salts such as aluminium, zirconium, titanium and iron are might be used as substitutes for chromium salts. However, under certain conditions aluminium is known to be more poisonous to aquatic life than trivalent and even hexavelant chromium. Re-use of chromium is a more realistic alternative (see par. 5.2.2). The unused tanning fluids which contain chromium can be collected separately. From these fluids and from the solids that contain chromium, chromium can be recovered. The remainder may be used as source material for glue and animal feedstuff. In countries where discharge of chromium is strictly prohibited, great efforts are made to recover and re-use chrome.
Alternative vegetable tanning methods can replace chrome tanning to a high degree. An example is the ‘Liritan’ process, developed in South Africa. A high chemical uptake, low pollution load, uniform penetration of the tan and a shortened process time with consequent financial efficiency are claimed to be the main advantages of this process (Higham, 1991), but little is known on the practical implications.
Finishing
A reduction of volatile organic compounds (VOC) can be accomplished by using aqueous finishes for base and middle finishing coatings.