F. Hontoria
INTRODUCTION
The nutritional requirements of marine organisms, and particularly the requirements of their larval stages, have been a point of major interest for the development of aquaculture. Presently, a number of essential nutrients have been identified. However, the techniques utilised to supply them could be improved in most cases. Our present work on liposomes suggests an alternative fro providing these nutrients as a mean to increase their efficiency. In our case, we study the delivery of polynsaturated fattyacids (HUFA) to marine fish larvae as an example for the use of this delivery system.
Liposome are artificial membranes composed of phospholipids that entrapaes an queous phase surrounded by a lipophillie matrix (figure 1). Among the liposomes different types can be distinguished: small unilamellar vesicules (SUV) with diameters below 100 mm, large unilamellar vesietes (LUV) With derneters above 100 mm, or multilamellar vesicules (MLV). It is interesting to note the differences with another structure that it is formed when lipids are dispersed in aqueous environments: the micelles. This structure is composed of a single layer of phospholipids and it does not allow the presence of the internal aqueous compartment. It is possible to form liposomes entrapping different nutrients. The use of liposomes has distinct advantages: slow and controlled release, in some cases, preferential accumulation in different tissues, and of particular interest in the case of dictary nutrienl delivery: additional protection against degradation, ability to trap either water soluble or lipophilic nutrients, and high versatility in composition, size and methods of formation.
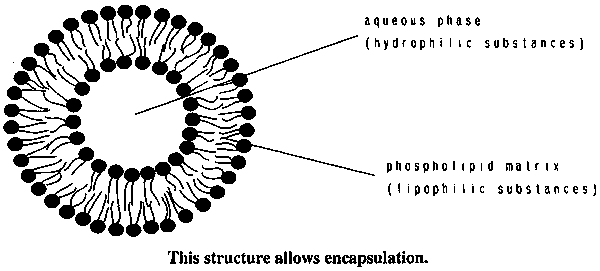
Figure 1. Liposome structure.
FORMATION AND STABILITY OF LIPOSOMES IN SEA WATER
Liposome systems have been extensively studied for its use in mannuals. This is not the case for aquatic environments, and specially for marine use. The formation and stabiity of liposomes in sea water have been explored.
The variation along time of the size of the formed population of liposomes (figure 2) is frequently utilised as an indicator of the occurrence of unstabilization processes (fusion, aggregation). In our case the trend of increase in mean diameter in all the preparations indicates the occurance of such processes, even with the additiopn of phosphatidylserine (PS), a phospholipid impaired with negative charge that contributes to the repulsion of liposome surfaces, thus preventing fusion and aggregation. However, the stability tests preformed (figure 3) show that the fusion among the dipalmitoil phosphatidyleholine (DPPC) liposomes is not likely occurring, since the fluorescent marker is retained above 95% for longer periods than the increase of diameter. Under these circumstances aggregation without internal leckage must be happening. This assumption has been lately confirmed through freeze fracture preparations of the samples. The aggregation observed, probably due in this case to the particular ionic balance in sea water, lends normally to fusion and consequent leakage of the liposome inner compartment content, but in our case, the aggregates are easily dispersed by hand shaking, which indicates the weak linkk between membrances, and does not result in fusion. These results demonstrate the fesibility of the use of liposomes in marine environment and the differences in stability related to their composition, when egg yolk phosphatidylcholine (egg PC) is utilised.
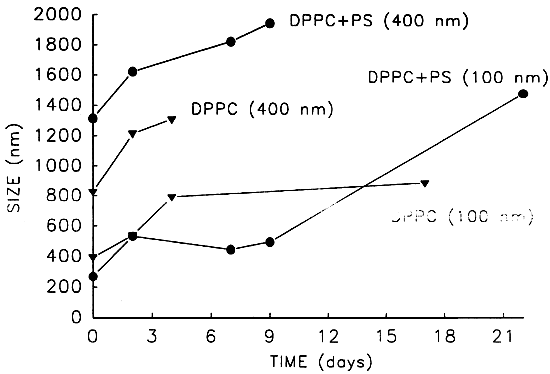
Figure 2. Variation of the mean size in liposome suspensions of different composition and extruded through different pore size (100 or 400 nm).
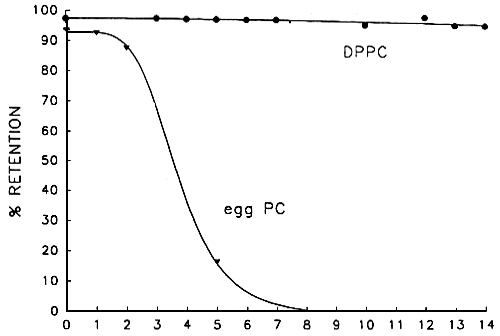
Figure 3. Retention of fluorescent market in liposomes of different composition.
HIGH PUFA CONTENT LIPOSOMES
The high and precise requirements of adequate levels of PUFA, especially those of the n-3 series, is presently fully established in marine fish larvae. The absence of these essential nutrients in the live preys utilised in larviculture has been overcome through different enrichment techniques, generally exposing live preys to fish oil emulsions. However, the difficulty of the enrichment process increases with the length of the fatty acid chain and there is some evidence that the phospholipid form and not the triglycerides used in emulsions, may be the preferred form in which the PUFA are assimilated. Because of the foregoing, the use of liposomes in this particular case can imporve the present PUFA delivery techniques.
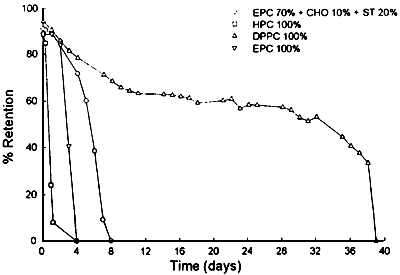
Figure 4. Retention of fluorescent market trapped inside liposomes with different composition. CHO, cholesterol: ST, stearyamine.
Our studies on the stability of liposomes formulated with herring PUFA rich phospholipids (HPC) indicate their fast degradation at room tempcrature (figure 4). However, increasing ratios of saturated phospholipids in the liposomes imporve their stability up to feasible levels for our purpose (figure 5). In addition, storage tests performed at 4°C show a long term stability of herring phospholipid liposomes (figure 6).
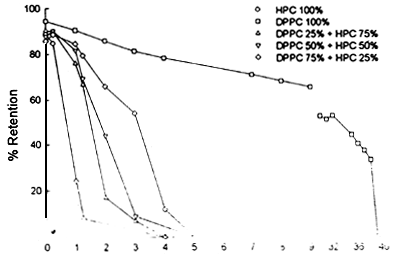
Figure 5. Retention of fluoresnt market trapped inside liposomes with differcut proportions of saturated phospholipids.
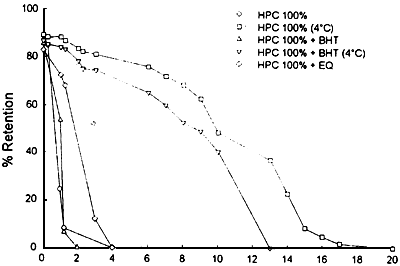
Figure 6. Retention of fluorescent market trapped inside liposomes stored at different temperatures with the addition of antioxidants.
LIPOSOME SUPPLEMENTATION TO LARVAE
There are three obvious ways to introduce the liposomes in the larvae:a) direct supplementation, b) biocecapsulation through live preys, and c) as part of microparticulated pellets.
The first option, though possible, seems the least realistic due to its economic limatations. Since maintaining the required liposome concentration in the larval rearing tanks would be highly expensive, and it is difficult to avoid mortality when larval density is increased. This is not the case with rotifers and Artemia nauplii. We have studied the bioencapsulation of fluorescence and radioactively (figure 7) marked liposomes in Artemia nauplii demonstrating its feasibility.
The third option requires the dehydration of the system that must maintain its integrity upon rehydration. Our results indicate that this is possible, if the liposomes are dehydrated in the presence of certain disaccharides (figure 8).
It is worthy to note that the liposome technique can be easily adapted to its use in other development stages, and that it is not necessarily constrained to the supplement of nutritional requirements Therapeutic, hormones, genetic material, etc. can also be entrapped in liposomes.
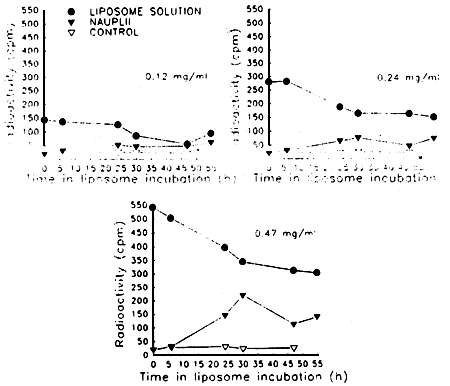
Figure 7. Accumulation of radioactivity in Artemia nauplii after incubation in 14 C-maltose marked liposomes at different concentrations.
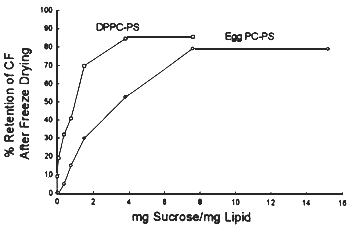
Figure 8. Retention of fluorescent maket trapped inside liposomes after freeze drying in the presence of different concentrations of sucrose.
Miquel Planas
Intituto de investigaciones Marines
Vigo, Spain.
Availability of fingerlings is the most critical factor in the commercial prodcution of fish farms. At hatching, most marine fish larvac, such as turbot, sea bass, sea bream or mahi mahi, have very limited yolk reserve lating for no longer than one to three days. In addition, at first feeding the mouth size is small. Therefore, the transition from endogenous to exogenous feeding is a very important problem with incidence on both survival and growth rates. First feeding must be perfectly established as soon as possible. This is particularly important in species where the yolk reserves are almost exhausted at the time of mouth opening. The main factors involved in the adequate feeding of larve are: temperature, light, prey characteristics and physiological or morphological alterations of the larvae (Figure 1). The effect of the temperature is also noticeable during the embryogenesis.
TEMPERATURE
Temperature has incidence on the conversion efficiency of the yolk both during embryogenesis and in yolk sac larvae (Table 1). As a result of high temperatures newly hatched larvae are longer and show bigger months at first feeding. Temperature also affects feeding incidence and ingestion rates. Consequently, growth and survival are also affected.
Figure 2 shows the effect of the tempertature on the feeding incidence of turbot larvae fed on four prey regimes: small rotifers (Bs), large rotifers (BI), Artemia nauplii (AN) and a mixture of these three prey (M). Feeding incidence was particulary affected by temperatura at first feeding and beyond day 6. A similar but more drastic effect was found for gut contents as shown in figure 3. However, the temperature affects to the larvae on a different way depending on the species (Figure 4). Turbol larvae are more active feeders than sea bream larvae and therefore the effect of temperature on feeding performaces is more drastic in sea bream.
LIGHT
Another important factor, Althought not well studied, is the light (Table 2). First, the type and intensity of light must be considered as they affect the perception of the prey by the larvae. Experiments carried out on sea bass demonstrated high mortalities under strong light intensities, particularly at the eleutheroembryo stage, when the larvae are younger than seven days and still not well pigmented. Also these parameters were found to be dependant on the photoperiod used. On this sense, it is suggested to test different light regimes and to analyse gut contents, survival and growth rates to establish optimal light conditions. Second, the level of the photoperiod is also important. Short photoperiods improve survival meanwhile large photoperiods improve growth and decrease functional swim bladder rates. It is proposed to establish the optimal photoperiod by testing different photoperiods and selecting the one giving the best overall production. It is very important to remember that light intensidy and photoperiod tests must not be carried out separately.
Figure 5A shows the improvement of the survival and condition index of sea bass larvae at day 30– 40 under low light intensities. The results obtained from two experiments performed on sea bass under different photoperiods are given in figures 5B. Long photoperiods were found to decrease survival rates and to improve the condition index. However, different results were obtained when photoperiod regimes and light intensities were studied simultaneously (Figure 5C). Highly significant survival rates can be obtained with large photoperiods (see the arrow in figure 5C) just by decreasing the light intensity before the total pigmentation of the larvae.
PREY CHARACTERISTICS
One of the most important factors in the feeding of larvase is the prey characteristics that interfere with growth and survival rates (Table 3). Larval metabolism is directly affected by the biochemical composition of the ingested prey. Directly implicated in the feeding incidence and ingestion rates of the larvae are other prey characteristics such as the size, type and density.
It has been reported that prey selection of fish larvae depends on the mouth size. In turbot, there is a clear relationship between prey width and both mouth wide (Figure 6: Left) and mouth height (Figure 6: Right) of the larvae. Prey with a width of about 40% mouth and 36% mouth height are preferentially selected. This is particularly important at first feeding when small rotifer strains improve the larval feeding performances, as shown in figure 7. This figure shows the results on the feeding performances (feeding incidence and gut content) of turbot larvae when fed on 4 types of diets. It is interesting in this figure the decrease in the feeding during the critical phase of mortality (about day 8-,5.3 mm standard length).
This kind of studies is useful to determine the optimal prey to use for each developmental stage of the larvae. Electivity indices (such as Ivlev electivity index) are also a good tool to study prey preferences.
MICROBIOLOGICAL ENVIRONMENT
More recent studies have been focused on the effect of the microbiologial environment on larval mortalities. It has been suggested that the prey is the main contamination source to the larvae, although the bacterial load of the surrounding water and of the detritus in the tanks is also important.
Bacteria can directly affect the gut of the larvae by producing diseases in the tissues and by changing the bacterial diversity (Table 4). Indirectly larval feeding and growth are also affected due to a decrcase in the feeding incidence and ingestion rates. Furthermore, survival rates probably decrease because of an increase in potentially pathogenic bacteria, mainly Vibrionaceae. These problems can not be avoided though reduced by decreasing the bacterial load in the prey and by applying rigorous cleaings.
Figure 8A shows the gut content of turbot larvae fed on different densities of rotiers. It is important to notice here the sudden decrease on gut contents at day 7. By this day, in one of he trials (trophic chain) some larvae with a very few prey in the gut were separated from others with very high gut contents. The guts of both groups of larvae were analysed for bacterial load and the results showed that the first group of larvae were completely invaded by bacteria. On the contrary, in the second group the bacteria were scare. From day 8, high numbers of bacteria in the larval guts were observed in all the trials together with a sharp drop in the gut content.
After this experiment, other rearing trials were carried out to test the effect of disinfection treatments (antibiotics): the larvae were treated 2 or 4 times during the first 10 days of rearing. The results (Figure 8B) show an important drop in the content of the larvae that received only two disintection treatments. This drop was located at day 6 (2 days before or after the treatments). This drop was not observed in the larvae that received 4 treatments. These results clearly show the negative effect of bacterial on the larval feeding.
Disinfecting treatments have also a positive effect on the rearing performances (larval growth and suvival) and reduce the variability of the results.
Consequently, the question is: How to reduce the bacterial load of the prey? This problem can partially be overcome by application of rigorous washings together with fresh water baths, especially after the enrichment (Table 5). In addition, short starvation periods before enrichment are also applied to rotifers in our laboratory. By this method, the rotifers empty the guts, and therefore the bacterial load decreases. The use of probiotics, which should be used instead of antibiotics, is also highly recommended. Today, new work is being made on the incorporation of therapeutics to the prey and larvae through oral administration of bioencapsulates.
SWIM BLADDER ABNORMALITIES
Among all the morphological or physiological alterations interfering with the larval feeding the problem of swim bladder inflation must be especially considered (Table 5). The absence of swim bladder inflation is due to the inhibition of the air-intake by physostomous larvae. To explain this fact three hypotheses have been proposed:
- A strong water current due to too vigorous aeration,
- A weak ability of the larvae, probably due to nutritional problems, or
- The formation of an oily film on the water surface.
The later hypothesis seems to be more likely.
On the contrary, a swim bladder hyperinflation is frequently observed under gas hypersaturation
conditions, mainly nitrogen. Therefore, the elimination of the oily film by a blower combined with
a floating trap degassing devices is highly recommended.
Some species are particularly affected by swim bladder problems. Sea bass and sea bream are some of them. (Table 6). The larvae lacking a swim bladder have buoyancy problem and extra effort to maintain a postition in the water column causes spinal deformities, poor growth and low feeding success. In the case of swim bladder hyperinflation, the larvae float near the water surface and stop feeding and swimming. These larvac will die in a few days due to starvation.
Figures 10A and 10B show the relationship between swim bladder inflation and growth of sea bream larvae. Figure 10A shows the results obtained (percentage of larvae with functional swim bladders) under different treatments.
- At day 20, name of the larvae reared in tanks which water surface was covered with paraffin developed the swim bladder.
- More than 80% of the larvae reared in the tanks in which a blower was used showed functional swim bladders. This percentage was significantly higher than in the tanks without blower (control).
The growths in length of these there groups of larvac were also significantly different (Figure 10B).
Finally, the information given in this paper must be considered as a general approach to the problematic of the feeding of marine fish larvae. The effect of environmental and biological parameters on the larvae is different according to the species considered. Therefore, the particular needs for each species must be stated by the corresponding studies.
RECOMMENDATIONS
The feeding performance of marine fish larvae can be improved by considering some general recommendations. The most important are the following.
Knowledge and control of the rearing conditions are very important. Special attention must be paid to temperatue, light, prey characteristic, microbiological environment, and factors involved in the occurrence of larval abnormalities.
Adequate tests should be continuously performed to modify and optimise the levels of the variables directly involved in the feeding performances of the larvae.
The optimisation of the larval feeding involves several variables that interest each other. Replicates and test repetition are very important to increase the reliability of the results.
Gut content analysis is a very simple tool in studying the feeding performances of the larvae.
The data given in scientific papers should be used as general guidelines but not as a “panacea”. Each experiment is carried out under particular and specific conditions, which probably are rather differente from ours.
EFFECTS OF THE TEMPERATURE
| • | Conversion efficiency index of yolk (embryogenesis and yolk-sac larvae) |
| • | Growth and survival |
| • | Mouth size at first feeding |
| • | Feeding incidence |
| • | Ingestion rates |
EFFECT OF THE LIGHT
| TYPE AND INTENSITY | |
| • | Affects perception of the prey by the larvae |
| • | Strong intensities could increase the mortalitics at particular developmental stages |
| • | The effect depends on the photoperiod utilised |
 | Test different light levels and analyse gut content, growth and survival of the larvae. |
| PHOTOPERIOD | |
| • | Short: Improves survival |
| • | Large: Improves growth. Low functional swim bladder rates |
 | Calculate their effects on the overall production and establish the ideal photoperiod for the production improvement |
 Light intensity and photoperiod studies must not be performed separately Light intensity and photoperiod studies must not be performed separately | |
EFFECT OF THE PREY CHARACTERISTICS
| SIZE, TYPE AND DENSITY | |
| • | Affects feeding incidence and ingestion rates |
| • | Consequently: affects survival and growth rates |
| BIOCHEMICAL COMPOSITION | |
| • | Affects larval metabolism |
| • | Consequently: affects survival and growth rates |
 Test different prey (e.g. rotifer strains) and enrichments whenever possible. Test different prey (e.g. rotifer strains) and enrichments whenever possible. | |
BACTERIA: THE EFFECT ON THE LARVAE
 | DIRECTLY TO: | |
| → | DIGESTIVE SYSTEM | |
| - desquamation of the epithelia | ||
| - necrosis | ||
| - changes in the bacterial diversity of the gut | ||
 | INDIRECTLY TO: | |
| → | LARVAL FEEDING AND CROWTH | |
| - decrease of feeding incidence and ingestion rates | ||
| → | SURVIVAL | |
| - increase in potentially pathogenic bacteria (mainly Vibrionaceae) | ||
  Reduction of the bacterial load in the prey Reduction of the bacterial load in the prey | ||
MORPHOLOGICAL ALTERATIONS: THE SWIM BLADDER
 | LARVAL FEEDING IS HIGHLY AFFECTED BY AN ABNORMAL DEVELOPMENT OF THE SWIM BLADDER | |||
 | TYPES OF SWIM BLADDER ABNORMALITIES | |||
| → | Uninflated swim bladder | |||
| (inhibition of air-intake by the larvae of physosteomous stage) | ||||
 | Cause: | 1- a strong water current by too vigorous aeration | ||
| 2- a weak ability of the larvae (low n-3 HUFA content in the prey) | ||||
| 3- the formation of an oil film on the water surface | ||||
| → | Hyper inflated swim bladder | |||
 | Cause: | 1- gase hyper saturation (N2) | ||
  | Continuous elimination of the superficial oily film by a blower combined with a floating trap. Use degassing devices. | |||
SWIM BLADDER ALTERATIONS
 | EFFECT ON THE LARVAF. (mainly S.aurta and D. labrax) | ||
| → | UNINFI.ATED SWIM BLADDER | ||
 | LORDOTIC DEFORMATIONS: | ||
| The larvae swim with an oblique direction of the body axis, in other to maintain a position in the water column. | |||
| Consequence: low feeding succes (low growth) | |||
| → | HYPERINFLATED SWIM BLADDER | ||
 | INACTIVE SWIMMING: | ||
| The larvae float near the water surface and stop feeding. | |||
| Consequence: larval death in few days. | |||
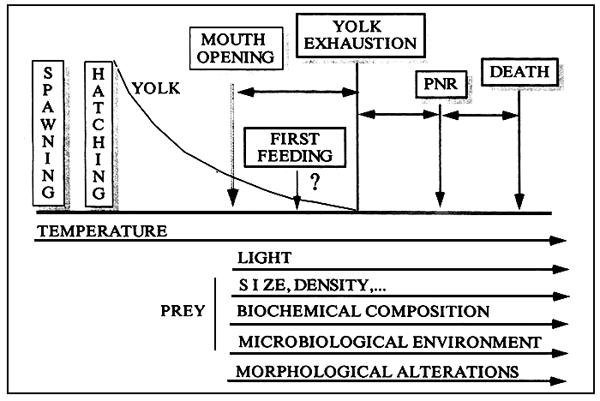
Figure 1
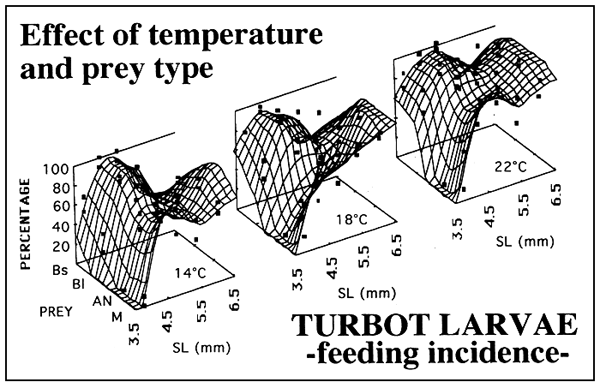
Figure 2
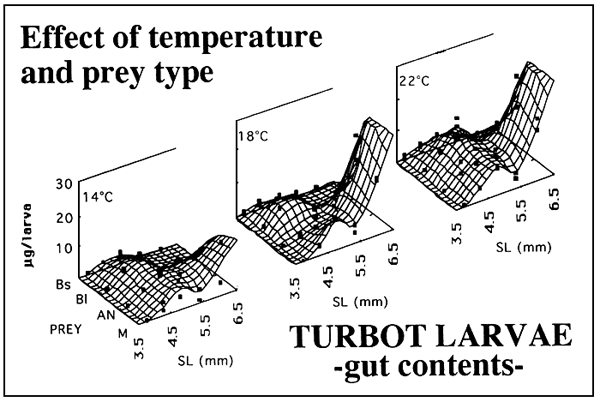
Figure 3
| Figure 4 | 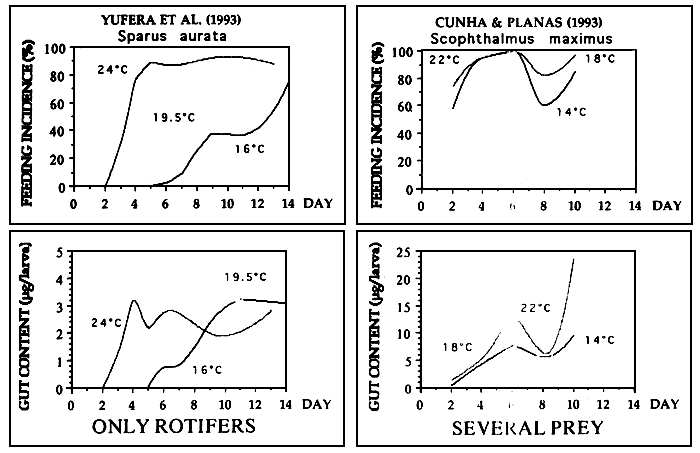 |
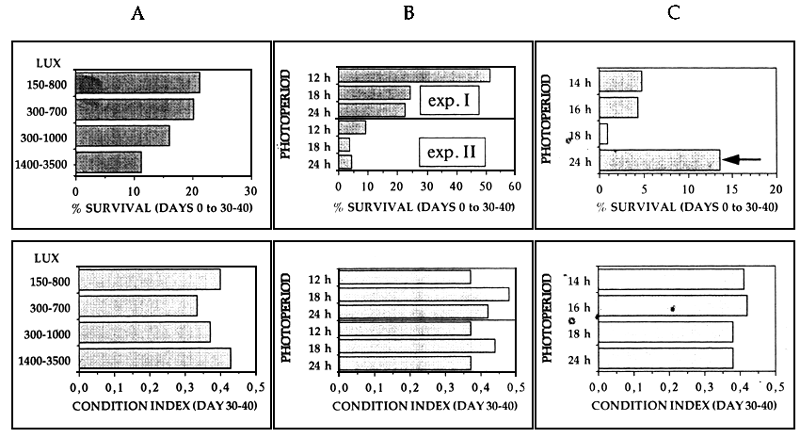
Figure 5
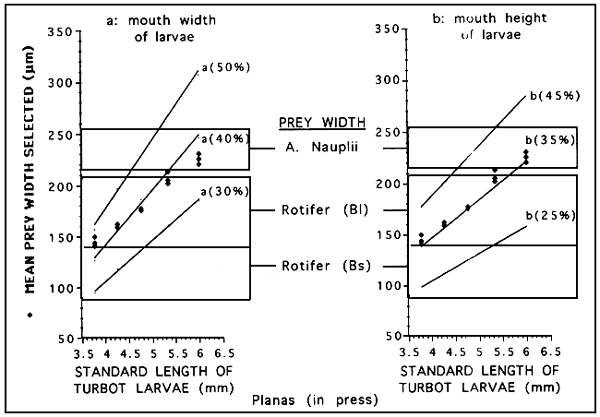
Figure 6
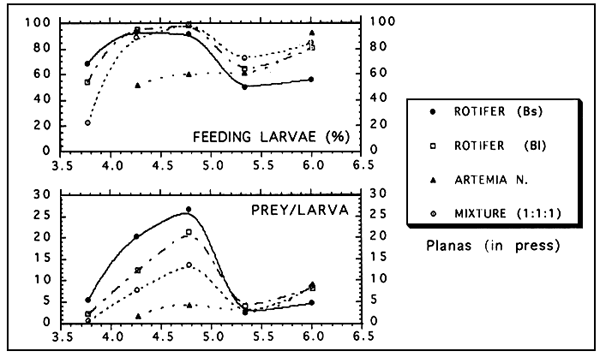
Figure 7
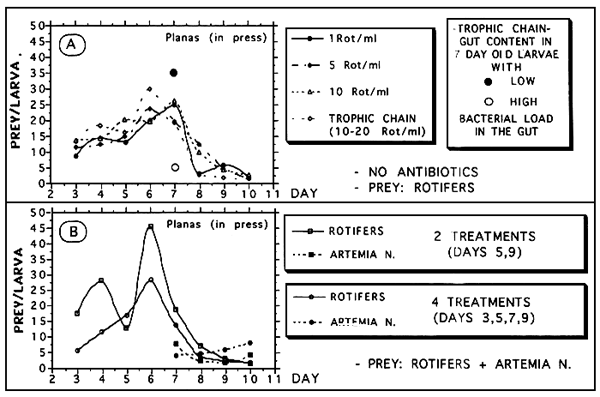
Figure 8
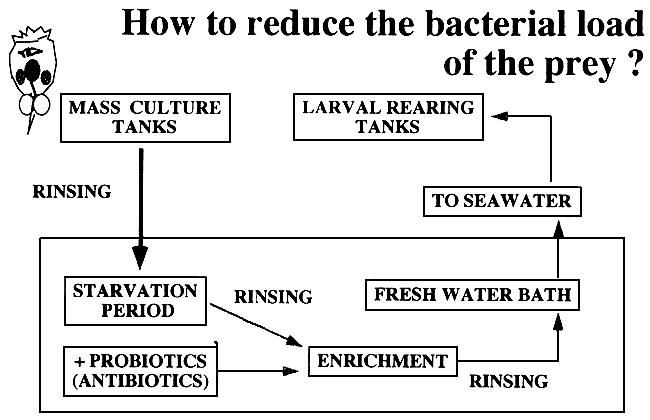
Figure 9
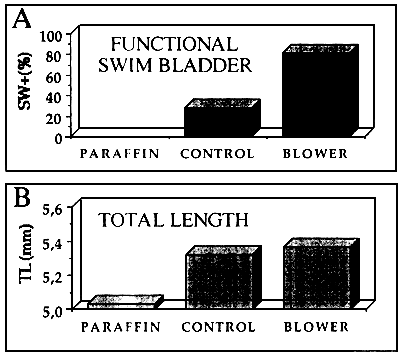
Figure 10
Jennine Person-le Ruyet
IFREMER - Laboratoire de Recherches Aquacoles
Plouzane (France)
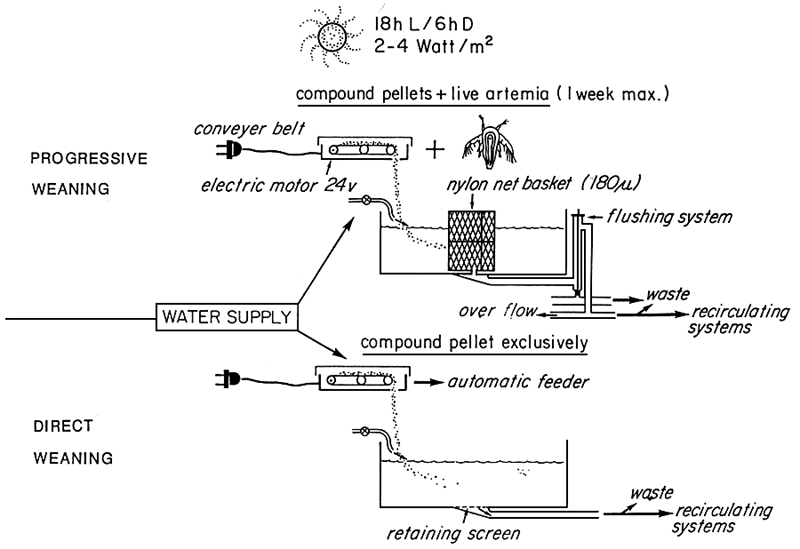
WEANING
| LIVE PREYS = | COMPOUND DIET |
| (artemia) | (pellet) |
WEANING CHARACTERISTICS
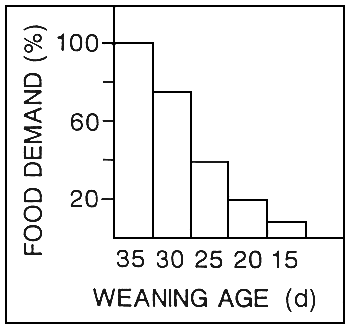
* AS EARLY AS POSSIBLE
WHY?
- live preys expensive
- nutritional quality unreliable (enrichment methods)
- sanitary value poor bacterial contamination)
* SHORT PERIOD
- 2 weeks (- 2–3 years --> market)
* INTENSIVE METHOD
- Controlled conditions
- high stocking densities
- method depending on weaning cage
WEANING STRATEGIES
| 1- LATE WEANING | Juveniles (> 1 month) | |
| (>50 mg) | ||
| 2- EARLY WEANING | Post-larvae (- D 20) | |
| (> 3 mg) | ||
| 3- START WEANING | Larvae (first feeding) | |
| (> 0,2 mg) |
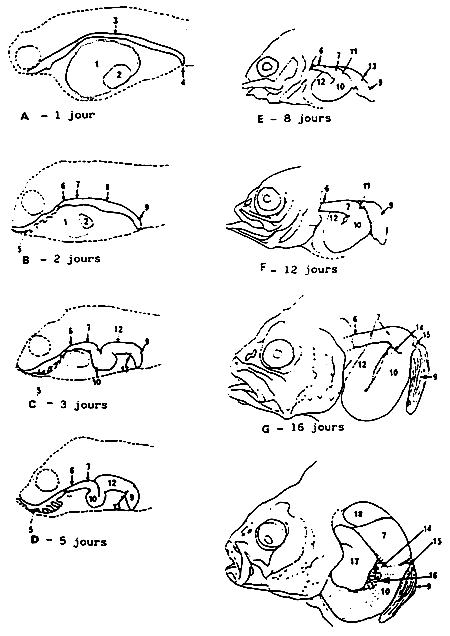
fig.1. Schéma de l'évolution du tube digestif. 1, Vitellus; goutte lipidique; 3, tube digestif; 4 ouverture anale; 5, cavité bucco-pharyngienne; 6, oesophage; 7, chez le turbot (cousin et el, 1986) estomac; 8, intestin antéro-moyen; 9, intensin postérieur; 10, intestin antérieur; 11, sphincter pylorique; 12, intestin moyen; 13, valve intensinale; 14, caecum pylorique ventral; 15, caecum pylorique dorsat; 16, pancréus exocrine; 17, foie; 18, vessie gazeuse.
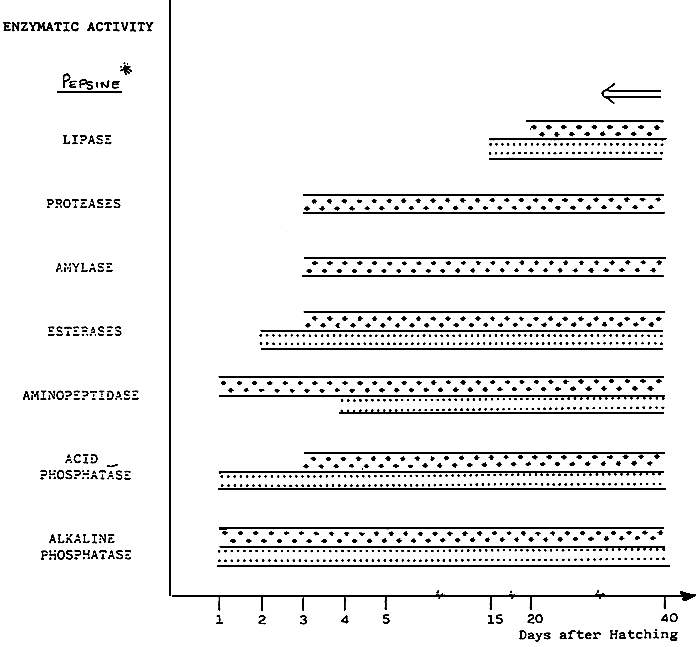
Table 2- Ontogenèse des activités enzymatiques mises en évidence par histo enzymologies chez le turbot (Cousin et al., 1986 sauf pour la pepsine, UBERSHAR 1985).
Ontogencsis of enzypatic activities demonstrated by histo enzyme chez le turbot (Cousin et al., 1986)* except for pepsine (UBERSHAR, 1985).
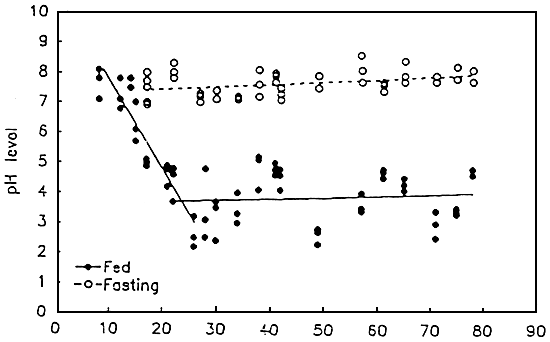
Fig. 3. The pH level in the anterior part of the digestive tube of fed and fastig seabass larvae and juveniles (measured in the presumptive stomach of early larvae before the stomach was formed and in the stomach of older larvae and juveniles). (Walford et Lam, 1993)
ENZYME SYNTHESIS
↓
ENZYME SECRETION
↓
HYDROLYSIS (INTESTINE LUMEN)
↓
ABSORPTION (THROUGH BRUSH BORDER)
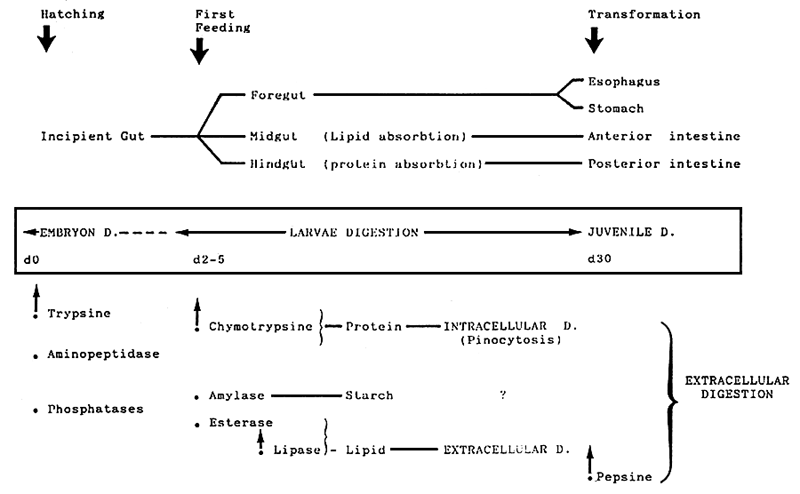
DIGESTIVE PHYSIOLOGY IN LARVAE
- ORGANOGENESIS OF DIGESTIVE TRACT
+++
- TIMING OF DIGSTIVE ENZYMES SYNTHESIS
* Pancreas ++
* gastric glands (+)
SECRETION CONTROL (PANCREAS INTESTINE)
?
- LEVEL OF ACTIVITY
* larvae age +
* diet +
* regulation ?
- ABSORPTION MECHANISMS
?
* intestinal enzymes of brush border
* active transport most often
* pynocytosis developped in larvae
SEVRAGE CLASSIQUE
- JUVENILE plan digestif
niveau d'alimentation satisfaisant
- ALIMENT SEVRAGE CONVENTIONNEL aliment juvénile
gamme de prix 10 à 60 F/Kg
SURVIE PENDANT LE SEVRAGE (BAR)
BASSINS DE 50 L
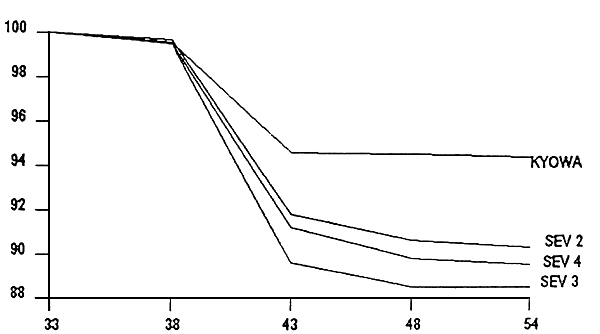
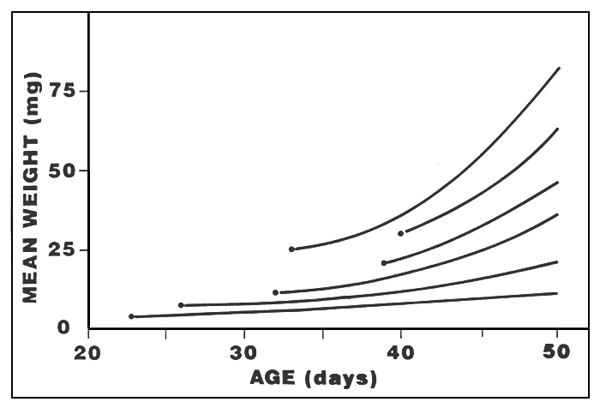
Figure 1- Evolution de la survie en fonction du régime alimentarie lors du sevrage en bassins de 50 l
CROISSANCE PENDANT LE SEVRAGE
BASSINS DE 50 L
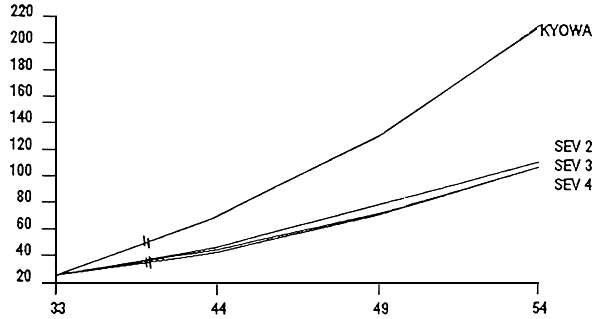
Figure 2- Evolution des poids moyens en fonction du régimealimentaire lors du swvrage en basins de 150 l
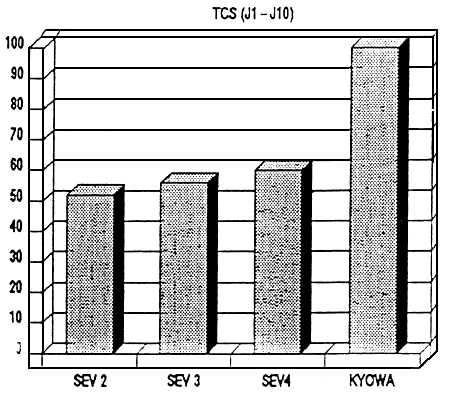
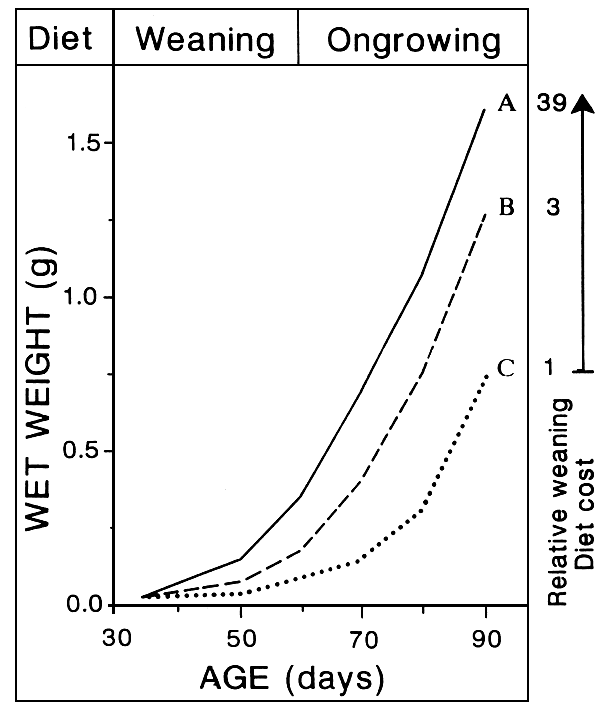
Figure 3- Videur relative du TCS les 10 premiers jours de sevrage
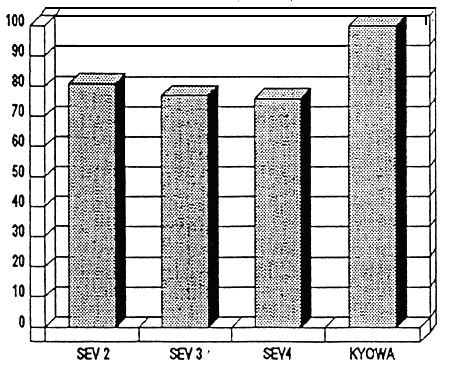
Figure 4- Valeur relative du TCS en fin de sevrage
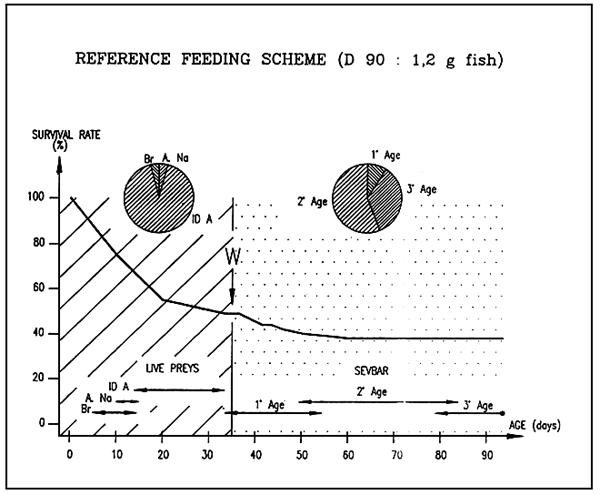
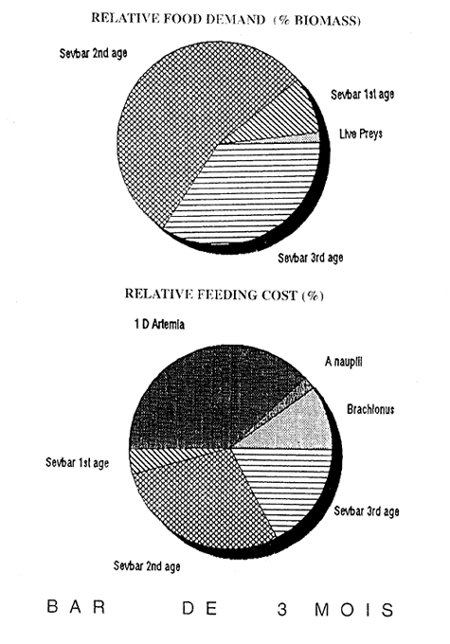
SEVRAGE CLASSIQUE DES POISSONS MARINS SUR SEVBAR
| ESPECE | AGE (JOURS) | POIDS (mg) | SURVIE% |
|---|---|---|---|
| BAR | 30 | 30 | 80 |
| DAURADE | 35 | 30 | 95 |
| TURBOT | 30 | 50 | 90 |
| SOLE | 35 | 75 | 80 |
| OMBRINE | 28 | 60 | |
| LATES | 25 | 80 |
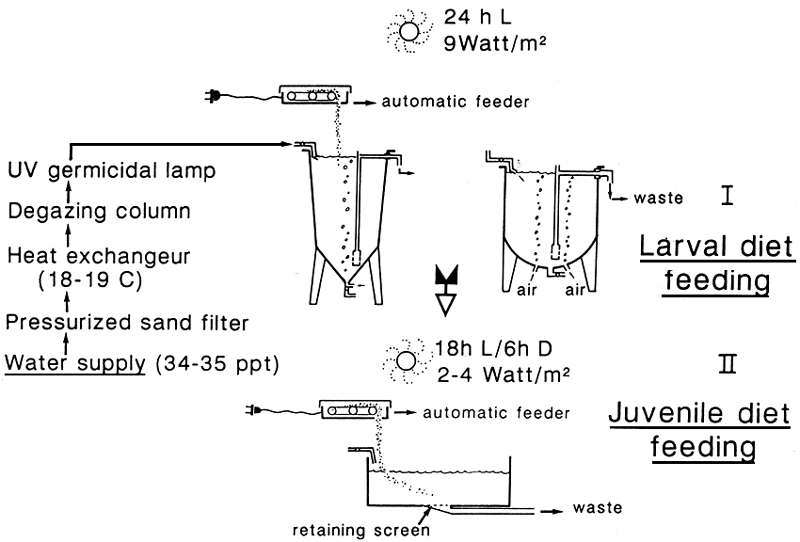
SEVRAGE AVANCE
- LARVE AGEE
3 – 5 mg
10 jours de réserves
niveau d'alimentation correct
- ALIMENT SPECIAL LARVE
microparticule, 125–200 um (substitut artemia)
protétines 60–65, lipides 20–25
matières premières d'exellente qualité
pocédé de fabrication spécial, “doux”
gamme de prix 300 à 600 F/Kg
2. CUMULATIVE MORTALITY
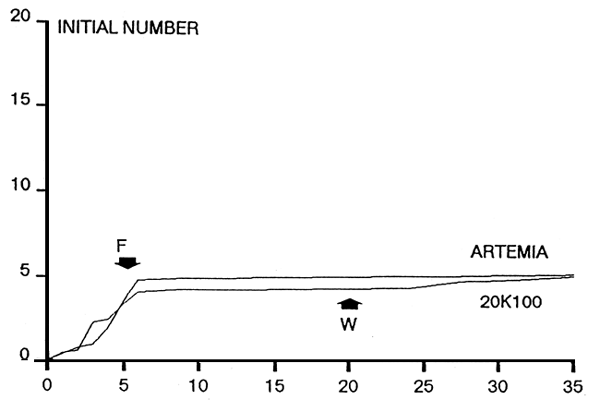
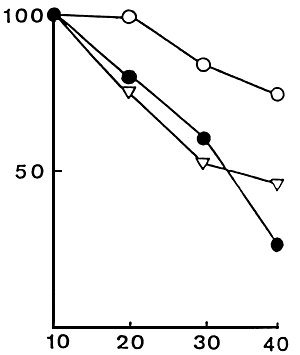 |  |
| TIME (days after hatching) | TIME (days after hatching) |
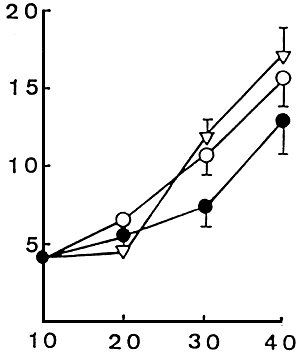
| Figure 1. Survival rates of red sea bram larvae fed microbound diets and live foods • : R1 (with soybean meal), O: R2 (Without soybeam meal), ∇: live foods. | Figure 2. Growth of red sea bream larvae fed microbound diets and live. • : R1 (With soybean mal). O:R2 (without soybean meal), ∇ : live foods. Values are mean total length (mm) + SE of the mean. |
EARLY WEANING
TECHNICALLY POSSIBLE
Up to 2 Weeks in advance
- FULL ARTEMIA SUBSTITUTIION
- CRITICAL AGE, RELANTED TO LARVAE STAGE OF DEVELOPMENT
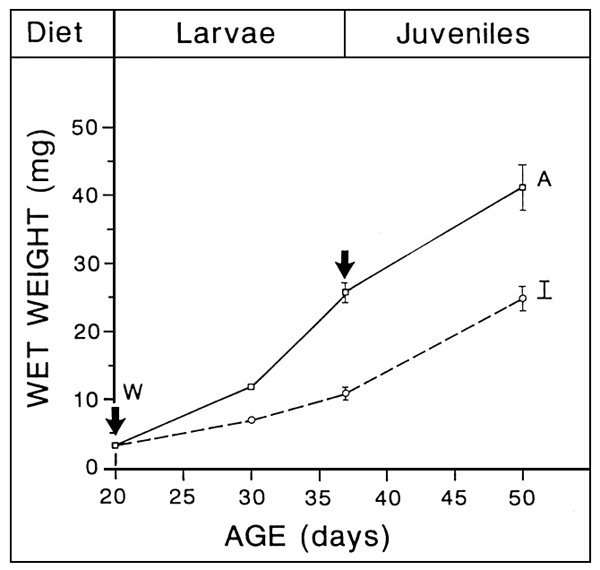
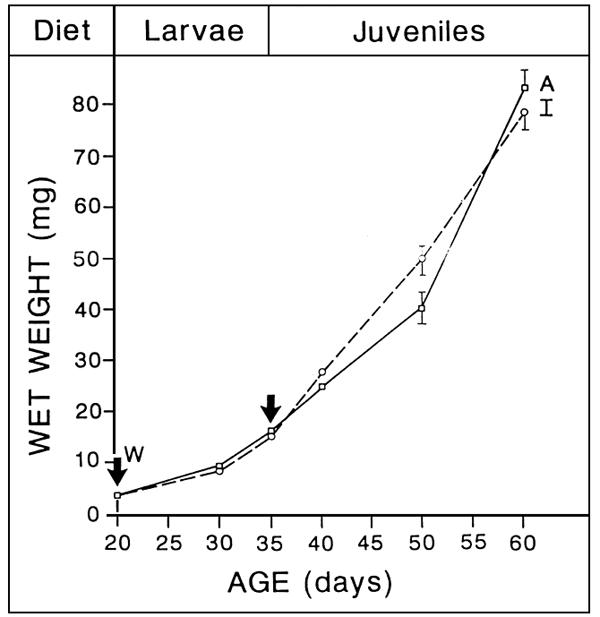
3 mg (D 15) in turbot?
- HIGH SURVIVAL
- ACCEPTABLE GROWTH RETARDATION
microparticles less efficient than live preys
- HIGH QUALITY JUVENILES
some risks of unreliability in juveniles quality
- MICROPARTICLES DIFFICULT TO USE
some risks of starvation and/or water pollution
- AVAIBILITY OF EFFICIENT COMMERCIAL MICROPARTICLES?
full substitution partial substitution
ALIMENTS AQUACOLES MICROPARTICULAIRES.
(d'après Teshima et al. 1982)
| Procédé | { | micro-texturisation (microbinding) | micro-encapsulation | micro-enrobage (microbinding) |
| Liant | { | Agar Agar | Nyon-protéine | Nylon-protéine |
| Gélatine | Gélatine-gomme arabique | Cholestérol-lécithine | ||
| Carraghénane | Chitosan | Zéine | ||
 |  |  | ||
| MBD | MED | MCD | ||
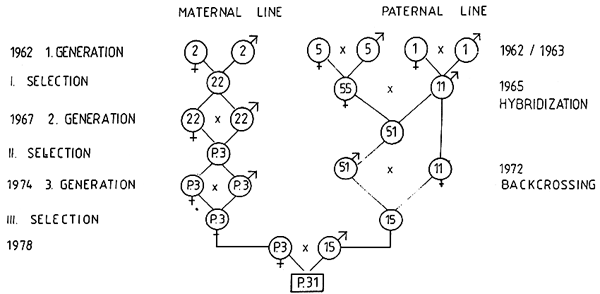
Figure. Te breeding scheme of the Szarvas P.31 three-line hybrid scaly carp
SEVRAGE PRECOCE
- LARVETRES JEUNE
0,3 mg
5 jours de réserves
- ALIMENT SPECIAL LARVE
microparticule, <200 um
encore plus élaborée (subsititut rotifèrc)
additifs éventucls
stimuler l'ingéré et les sécrétions enzymatiques?
jusqu'à 1300 F/Kg
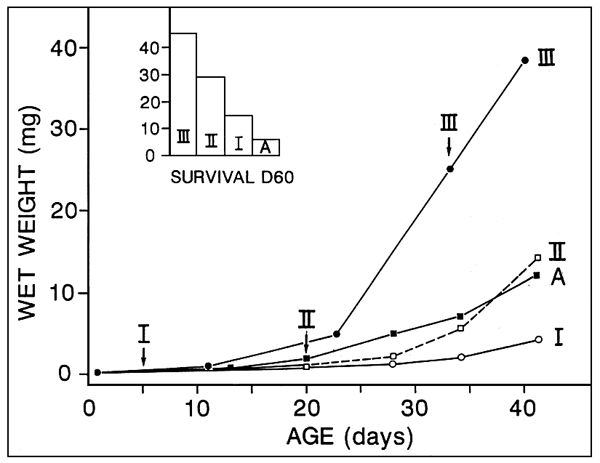
Figure 1
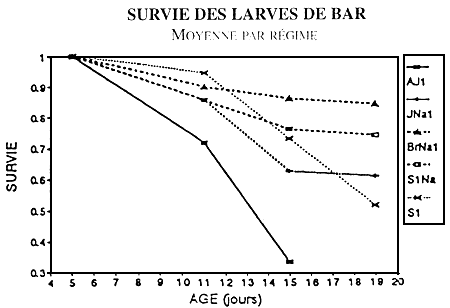
Figure 2
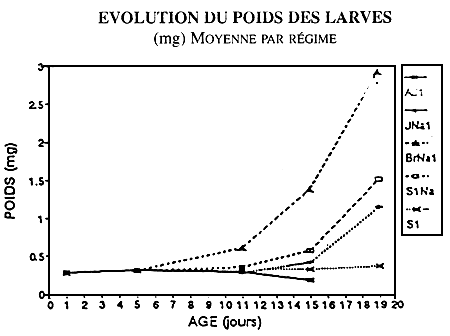
Table 1- Some examples of early weaning results
| Species | Length | Weight | Age | Diets | Results | Authors | |
| (mm) | (mg) | (d) | exp | Commercial | |||
| Dicentrurchus labrax | 4 | 0,2 | 5 | + | + | Poor, full Substitution | CAVALIER (1989) |
| 9 | 3 | 20 | + | + | High, full substitution | These results | |
| Sparus auata | 3 | 0,1 | 3 | + | High, half substitution | VERGARA MARTIN et al. (1990) | |
| 7 | 1,0 | 25 | + | High, half subsitution | CORNELLIE et al. (1989) | ||
| - | - | 20 | + | High. 80% subsitution | TANDLER et KOLKOVSKI (1991) | ||
| Solea solen | 4 | 0,4 | 2 | + | Poor, full substitution | APPELBAUM (1985) | |
| 7 | 3 | 10 | + | High, full substution | APELBAUM (1985) | ||
| 3 | 10 | + | High, full substution | GATESOUPE (1983) | |||
| Gadus morhua | 4 | 0,6 | 4 | + | Poor, full substitution | OPSTAD et al. (1985) | |
| Pagrus major | 4 | - | 10 | + | High, 90% substitution | KANAZAWA et al. (1989) | |
| Paralichithys olivanceus | 5 | 10 | + | High, 90% substitution | KANAAWA et al. (1989) | ||
| Sciaenops ocellatus | 3 | - | 5 | + | + | High, part substitutions | HOLT (1991) |
| Lates calcarifer | 2 | 2 | + | Poor, full substitions | WALFORD et al. (1991) | ||
| High, part substitution | WALFORD et al. (1991) | ||||||
START WEANING
- DIRECT WEANING AT FIRST FEEDING IMPOSSIBLE
- 2 ALTERNATIVE:
LIVE PREYS SUPPLEMENTATION
PREFEEDING LIVE PREYS PERIOD AS SHORT AS POSSIBLE
(up to a critical weight for carly weaning)
FEEDING STRATEGIES
- START WEANING IMPOSSIBLI:
- ROTIEFERS & ARTEMLA PARTIAL SUBSTITUTION,
2nd WEEK, POSSIBLE but VERY DIFFICULT
- EARLY WEANING PAST A CRITICAL SIZE POSSIBLE
FULL ARTEMIA SUBSTITUTION
PARTIAL ARTEMIA SUSBTITITION
- LATE WEANING VERY EASY