Table. 8. Utilisation of World Fishery resources for fish meal and fish oil production 1
| UTILIZATION OF WORLD FISHERY RESOURCES AND FISHMELL PRODUCTION 1984–1990 (values given in 000mt) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total world landing 2 | 83,925 | 83,378 | 92,825 | 94,399 | 99,062 | 100,333 | 97,246 | |
| - For human consumption | 58,475 | 60,090 | 63,990 | 66,571 | 69,819 | 70,905 | 70,212 | |
| - % total Landings | 69,7% | 69,6% | 69,0% | 70,5% | 70,5% | 70,7% | 72,2% | |
| - For other purposes | 25,450 | 26,288 | 28,839 | 27,828 | 29,243 | 29,428 | 27,034 | |
| Redaction 3 | 24,100 | 25,088 | 37,239 | 26,278 | 27,593 | 27,828 | 25,534 | |
| - % total Landings | 28,7% | 29,0% | 29,3% | 27,8% | 27,8% | 27,7% | 29,3% | |
| - % total catch | 31,2% | 31,9% | 32,4% | 31,2% | 31,4% | 31,3% | 30,0% | |
| Miscelan purposes | 1,35 | 1,200 | 1,600 | 1,550 | 1,650 | 1,600 | 1,500 | |
| Total fishmeal production 3 | 60119 | 6,343 | 6,723 | 6,509 | 6,846 | 6,897 | 6,293 | |
| - Fishmeal from white-fish | 239 | 217 | 190 | 187 | 198 | 192 | 154 | |
| - % total fishmeal | 3,9% | 3,4% | 2,8% | 2,9% | 2,8% | 2,4% | ||
| Make meal | 2,1 | 0,36 | 4,2 | 22,8 | 39,6 | 41,4 | 21,2 | |
| Other white-fish | 213 | 197 | 176 | 153 | 146 | 141 | 121 | |
| - Fishmeal form oily fish | 5,590 | 5,823 | 6,321 | 6,093 | 6,399 | 6,451 | 5,919 | |
| % total fishmeal | 91,4% | 91,0% | 94,0% | 93,6% | 93,5% | 93,5% | 94,1% | |
| Anchoveta meal | 208 | 313 | 745 | 499 | 648 | 835 | 592 | |
| jack mackerel meal | 324 | 332 | 256 | 399 | 446 | 510 | 529 | |
| plichard meal | 873 | 934 | 860 | 706 | 432 | 461 | 293 | |
| Menhaden meal | 286 | 279 | 269 | 303 | 299 | 210 | 203 | |
| Capel in meal | 133 | 144 | 144 | 126 | 161 | 112 | 117 | |
| Tuna meal | 43,6 | 38,6 | 41,5 | 54,1 | 53,3 | 48,4 | 41,4 | |
| Herring meal | 30,8 | 24,3 | 24,3 | 29,3 | 37,2 | 34,3 | 41,8 | |
| Clupeoid fish meal | 38,1 | 35,1 | 33,1 | 36,5 | 37,2 | 59,5 | 62,5 | |
| Oily fish meal (others) | 3,615 | 3,711 | 3,940 | 3,926 | 4,321 | 4,166 | 3,988 | |
| - Miscellaneous meals 4 | 21,2 | 15,9 | 19,2 | 25,8 | 25,9 | 28,4 | 26,9 | |
| - % total fismeal | 0,3% | 0,2% | 0,3% | 0,4% | 0,4% | 0,4% | 0,4% | |
| - Fish solubles | 268 | 286 | 192 | 203 | 223 | 225 | 193 | |
| - % total fishmeal | 4,4% | 4,5% | 2,9% | 3,1% | 3,3% | 3,3% | 3,1% | |
| Total fishmeal exports | 2,654 | 3,120 | 3,264 | 3,286 | 3,268 | 3,754 | 3,220 | |
| - Value 1,000,000us $ | 1,036 | 933 | 1,108 | 1,230 | 1,622 | 1,642 | 1,441 | |
| Total oul & fat production 5 | 1,520 | 1,496 | 1,671 | 1,453 | 1,565 | 1,639 | 1,398 | |
| - Fish liver oils | 30,0 | 31,3 | 24,6 | 34,0 | 38,8 | 31,2 | 23,9 | |
| - Fish oils & fats (others) | 1,481 | 1,454 | 1,641 | 1,415 | 1,524 | 1,602 | 1,368 | |
| Total oil & fat exports | 945 | 991 | 807 | 688 | 835 | 798 | 737 | |
| - Value 1,000,000us $ | 329 | 298 | 205 | 183 | 294 | 195 | 206 | |
1 FAO (1992b). The total landings of fish and shellfish in 1991 are reported to be 96,925,900 mt (FAO, unpublished data)
2 Total world landings includes fish and shellfish, and excludes whales, seals, other aquatic mammals and aquatic plants
3 Only whole fish destined for the manufacture of oil and meals is included.
4 Miscellaneous meals of aquatic or origin include crab meal, shrimp meal and crustacean meals
5 Oils and fats, crude or refined, of aquatic animal origin
Table 9. Top twenty fish meal producing countries in 1990 1
| TOP 20 FISHMEAL PRODUCING COUNTRIES IN 1990 (values in '000 metric tonnes) | ||
| COUNTRY | PRODUCTION (MT) | EXPORTED % |
| 1. PERU | 1,204 | 94.2 |
| 2. CHILE | 1,073 | 92.0 |
| 3. JAPAN | 977 | 15.1 |
| 4. USSR | 695 | 1.2 |
| 5. USE | 354 | 17.0 |
| 6. THAILAND | 265 | 5.8 |
| 7. DENMARK | 260 | 66.0 |
| 8. NORWAY | 184 | 24.7 |
| 9. ICELAND | 150 | 82.7 |
| 10. SPAIN | 135 | 17.5 |
| 11. MEXICO | 83 | 0 |
| 12. CHINA | 79 | 11.4 |
| 13. CANADA | 68 | 39.8 |
| 14. ECUADOR | 59 | 34.2 |
| 15. INDIA | 58 | 0 |
| 16. SOUTH AFRICA | 54 | 0.2 |
| 17. UK | 51 | 10.7 |
| 18. KOREA REP | 50 | 10.0 |
| 19. TURKEY | 50 | 0 |
| 20. MALAYSIA | 43 | 19.5 |
| Total | 5,892 | |
| World total | 6.293 | |
| Top 20 as percent of world total | 93,6% | |
TABLE 10. Observed dietary inclusion level (%) of selected feed ingredients commonly used within pratical complete diets for warmwater fish species, and suggestions for their maximum dietary inclusiton level 1
| Feed Ingredient | Carnivorous | Omnivorous herbivorous | ||||
|---|---|---|---|---|---|---|
| Range | Mean | Max | Range | Mean | Max | |
| Alfelfa meal 1-00-023 2 | 1–5 | 3 | 5 | 3–60 | 22 | 30 |
| Blood meal 5-00-381 3 | 2–30 | 9 | 15 | 1–50 | 12 | 20 |
| Brewers grains 5-02-141 | 5–15 | 10 | 15 | 5–86 | 25 | 35 |
| Copra meal 5-01-572/3 4 | - | - | 15 | 7–33 | 18 | 25 |
| Corn grain 4-02-935 5 | 2–15 | 8 | 20 | 10–33 | 26 | 35 |
| Corn gluten meal 5-28-241 | 4–20 | 10 | 15 | 4–17 | 10 | 20 |
| Corn DGS 5-28-236 6 | - | - | 15 | 10–70 | 35 | 35 |
| Corn DDS 5-28-237 7 | 3–8 | 7 | 10 | 5–8 | 6 | 15 |
| Cottonseed meal 501617/218 | - | - | 15 | 7–80 | 25 | 35 |
| Feather meal 5-03-795 9 | 3–55 | 7 | 15 | 2–21 | 9 | 20 |
| Fish meal (general) | 25–75 | 50 | 80 | 0–60 | 25 | 80 |
| Fish protein concentrate | 5–10 | 8 | 15 | 2–8 | 3 | 10 |
| Kritt meal 5-16-423 10 | 5–20 | 10 | 25 | - | - | 25 |
| Linseed meal 5-02-045/8 11 | - | - | 15 | 10–88 | 25 | 35 |
| Liver meal 5-00-389 | 5–65 | 25 | 50 | 5–45 | 20 | 50 |
| Meat & bone meal 5-00-38812 | 5–30 | 10 | 25 | 3.78 | 15 | 35 |
| Peanut meal 5-03-649/50 13 | 5–20 | 10 | 15 | 4–80 | 25 | 35 |
| Poultry meal 5-03-798 14 | 10–46 | 15 | 25 | 5–25 | 10 | 35 |
| Rapeseed meal 5-03870/1 15 | 10–30 | 15 | 20 | 5–63 | 25 | 35 |
| Rice bran 4-03-928 16 | 5–15 | 10 | 15 | 3.75 | 25 | 35 |
| Shrimp meal 5-04-226 17 | 5–30 | 10 | 25 | 5–15 | 7 | 25 |
| Sesame seed 5-04 220 18 | - | - | 15 | 10–78 | 25 | 35 |
| Sorghum grain 4-04-383 19 | - | - | 20 | 10–57 | 18 | 35 |
| Soybean meal 5-20-637 20 | 6–45 | 15 | 25 | 4–68 | 25 | 40 |
| Soybean aeeds 5-04-597 21 | 10–30 | 15 | 25 | 10–58 | 30 | 40 |
| Squid meal 5-04-671 | 5–30 | 10 | 50 | - | - | 50 |
| Sunflower sed 5-4-738/9 22 | - | - | 15 | 10–70 | 26 | 35 |
| Wheat grain 4-05-268 23 | 4–33 | 15 | 20 | 4.25 | 15 | 35 |
| Wheat bran 4-05-190 | 2–25 | 10 | 15 | 10–50 | 25 | 35 |
| Wheat gluten meal | 5–10 | 7 | 15 | 2–10 | 5 | 15 |
| Wheat middlings 4-05-205 | 2–38 | 16 | 25 | 2–42 | 20 | 40 |
| Yeast 7-05-527/34 24 | 2–62 | 15 | 50 | 5–56 | 10 | 50 |
4- Copra meal protein is deficient in methionine and lysine
6- Corn Distillers Grains with Solubles
7- Corn Distillers Dried Solubles
10- Dietary inclusion level limited by fluoride content of the meal
17- Dietary inclusion level is normally limited by the high crude fibre content of the meal
21- Heat processed full-fat soybeans
Table 11 : Reported chemical analysis of fish meals produced from whole herring of different degrees of freshness (from Pike, 1991 a)
| RAW MATERIAL | FRESH | MODERALTELY | STATE |
|---|---|---|---|
| TVN in raw material | |||
| mgN/100g | 22 | 62 | 143 |
| Fish meal | |||
| Protein% | 73,5 | 73,1 | 69,4 |
| Oil% | 18,7 | 8,1 | 10,9 |
| NH3-N% | 0,12 | 0,16 | 0,25 |
| Cadaverine ppm | 330 | 1000 | 1600 |
| Putrescine ppm | 30 | 230 | 630 |
| Huistamine ppm | <30 | 440 | 830 |
| Tyramine ppm | <30 | 400 | 800 |
1 Iced raw material processed (low-temperature drier) within 12 hours of catching (fresh), after two to three days of storage (moderately fresh) and after week storage (state)
Table 12 : Quality Standars for Soybean
| a) Specification of soy products (Ntional Soybean processors Association) | |||
| Soybean Cake | Soybean Meal | Dehuilled soybean | |
| Protein, minimum | 41,0% | 44,0% | 48,0% |
| Fat, minimum | 3,5% | 0,5% | 0,5% |
| Fibre, minimum | 6,5% | 7,0% | 3,5% |
| Moisture, maximum | 12,5% | 12,0% | 12,0% |
| Urease activity | |||
| (range : increase in Ph) | 0,05–0,20 | 0,50–0,20 | 0,50–0,20 |
| b) Correlation between urease activity (Ph change), protein solubility index (percent), and trypsin inhibitor (mg/gm) meal) in soybean meal | |||
| Urease activity | Protein solubility | Trypsin Inihibitor | |
| 2,40 | 99,2 | 21,0 | |
| 2,04 | 87,7 | 12,2 | |
| 0,23 | 79,1 | 3,1 | |
| 0,12 | 83,2 | 2,2 | |
| 0,00 | 74,9 | 2,1 | |
| 0,00 | 71,8 | 1,0 | |
| 0,00 | 58,5 | 0,5 | |
| 0,00 | 38,0 | 0,1 | |
1 From Akiayama (1988)
ANNUAL WORLD LANDINGS OF FISH AND SHELLFISH
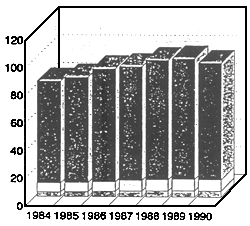
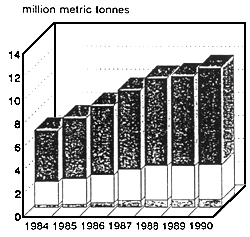
Figure 2: Total World aquaculture production in 1990 (1)
Total aquaculture production - animals & plants 15,322,703 mt
Total value of aquaculture production - 26,5 billion US $
(1) FAO 1992
Figure 3 : Temperature and feeding habit of farmed fish in 1990
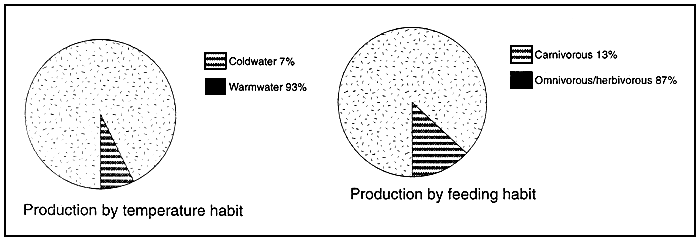
Coldwater fish species include salmons, trouts, smelts & cod
Carnivours fish species include the majority of marine finfish (except mullets), diadromous finfish
(except milkfish), snakeheads, certain catfishes and mollusc eating freshwater fishes
Figure 4 : World fish and crustacean aquaculture production in 1990 (1)
(values given in metric tonnes and as % of total.)
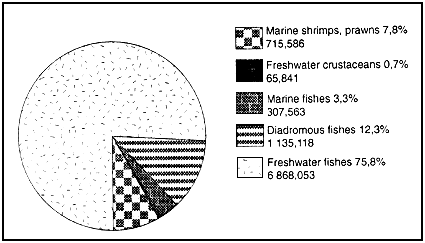
Total fish & crustacean aquaculture production 9 192,161 mt
(1) FAO 1992
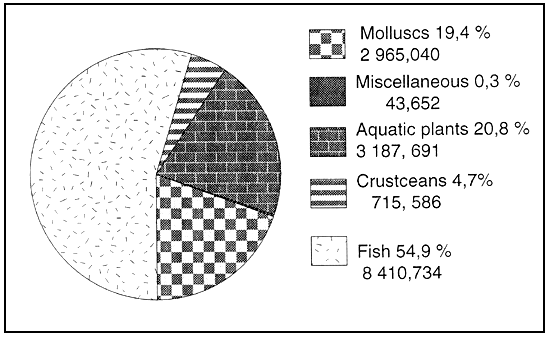
Figure 5 : World aquaculture production of fish in 1990 (1) (values given in metric tonnes and as % of total)
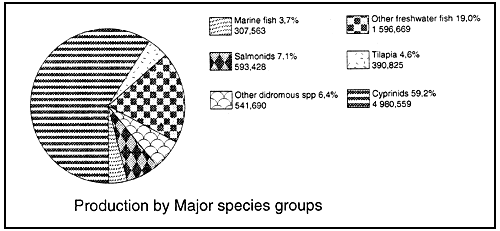
Total fish production 8 410,734 mt
(1) FAO 1992
Figure 6 : Top ten cultured fish and shrimp species in 1990 1/ (values given in metric tonnes and as % of total)
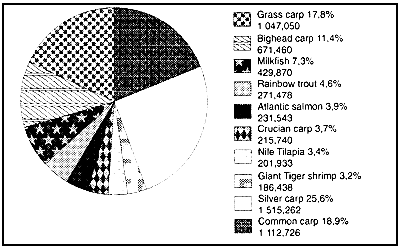
Total production 5 883,500 mt or 64,5% of all cultured fish and crustaceans
(1) FAO 1992
Figure 7 : Global use of fish meal in livestock and aquafeeds 1/ (values given as % and metric tonnes)
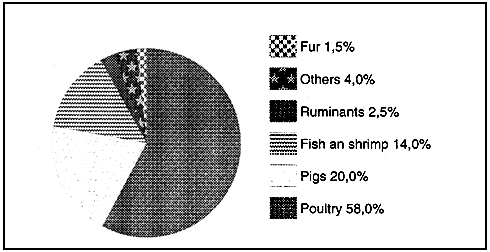
Fish meal consumption by animal species in 1990
(1) Pike 1991
Figure 8 : Fish meal consumption by aquaculture species in 1990 1/ (values given as % and 000 metric tonnes)
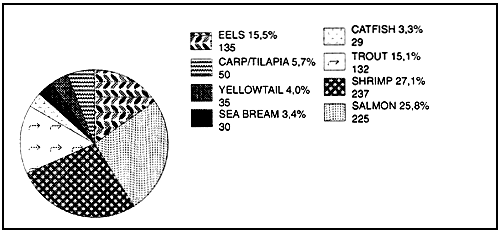
Total reported fish meal used within aquafeeds 873,000 mt
(1) Pike (1991)
Figure 9 : Fish meal consumption by aquaculture species in 1991 (1)
(values given as % and 000 metric tonnes)
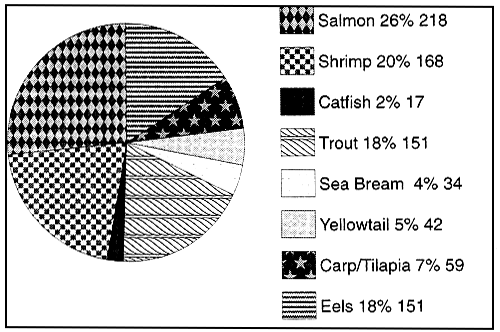
Total reported fish meal used within aquafeeds 840,000 mt
(1) Spingate & Gallimore (1992)
Figure 10 : Fish meal consumption by aquaculture species in 1992 (1)
(values given as % and 000 metric tonnes)
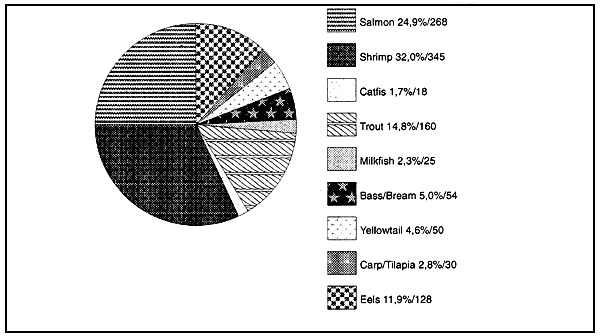
Total reported fish meal used within aquafeeds 1 078,000 mt
(1) I.H. Pike, Personal communication, IFOMA March 1993
Nicolas ROBERT
This talk will deal with bass and bream species.
First, it is necessary to remind some general feature of :
- Feed composition
- Energetic coefficient
- Digestibility
| Feed contains energetic components | : | (Protein, fat, carbohydrate and) |
| non energetic components | : | (ash, moisture, vitamines…) |
Each of the three energetic components has a specific energy value.
| Carbohydrate | : | 1 g provide 4.10 Kcal |
| Protein | : | 1 g provide 5.65 Kcal |
| Lipids | : | 1 g provide 9.45 Kcal |
Lipids which are very well digested are a very good energy dense source, approximately twice more
concentrated than protein. Carbohydrates have usually to be treated to be well digested by the fish
we are interested in.
These values are raw energy values. They have to be multiplied by the digestibility coefficient to
give the energy available for the fish.
ADC or apparent digestibility coefficient is the fraction of a component which will go through the
gut wall in the blood and lymph, and hence is available for fish metabolism.
Metabolism of the three energetic components.
In the stomach, the feed became a paste and is partially hydrolysed by acids and enzymes. This phenomenon
goes on the anterior gut with different pH and enzymes.
Proteins which are long polymers (chain) of Amino Acids are cut in smaller fractions which can go
through the gut wall, the same for carbohydrates which are polymers of oses (sugar).
Fat : triglycerids are separated in glycerol and fatty acids which goes easily through gut wall.
These energetic components present in the blood will have different pathways according to the ratio between Amino Acids and the energy given by the total (Amino Acids + sugars + lipids).
Amino Acid can follow two path :
If the energy needs are covered by lipids or/land sugars, a minimum part of them will be destroyed (deaminated) and a maximum part will be resynthetysed in fish protein and then stored as fish flesh.
But if energy brought by lipids + sugars is insufficient for maintenance, swimming… needs, then a
greater part of Amino Acids will be used as energetic component and deaminated : it means a loss
of nitrogen, transferred to water as a Ammonia, so that the remaining Acid can enter the energy producing
pathway of sugars.
It this case : protein is loosed and Ammonia discharge is increased, lipids will be stored according
to these two Amino Acids pathways.
They can provide energy through destruction into Co2 + H2O or they can be stored in fat issue as soon as energy needs are fulfilled.
So for each species and for each size of fish, we have to find the optima digestible protein/metabolisabe digestible energy which definition is :
- maximum protein efficiency - minimum nitrogen discharge
- optimum fat deposit (according to market or processing).
We see here that we can define a nutritionist optimal P/E ratio and an economical P/E.
If protein is in excess to energy then occurs a loss of protein so an increase in nitrogen discharge and cost, but in case of excess feeding, fat deposit can occur. If protein is too low compared to energy then you get a fatty fish because protein is a limiting factor.
It must be clear that even with high protein diet you can get a fatty fish according to your way of feeding.
In following text, number in ratio as 46/12 will express protein/fat content of the feed in % of raw weight.
Historical P/fat evolution (bass - bream - grower)
In the past, warm water marine fish were fed with low energy feed 45/9 : mainly uncoated feed (it
means almost without oil added).
Fat content was due to fish meal oil content.
Then coating with oil rise the fat content to 12 and now 15 for seabream, 20 for seabass.
Evolution of energy is given beside.
Protein level is quite stable but it has to be known that fish meal quality was improved meanwhile.
P/E requirement varies with size of fish and fish species larvae and fry need high protein content due to their very high rate of protein synthesis and turnover (and growth).
Then evolution from 115 to 102 mg prot/kcal is for a standard range of feed (46/12 as grower feed).
The point 45/15 (= 97 mg Prot/Kcal) is where we are now for seabream for instance.
As reference the pattern is given for trout range feed.
Warnings
The following results are field result, achieved on large production. They compare different commercial fees with different formulation and recipes.
The size of the fish analysed is marketable size and so is very different to fish size commonly used in nutrition scientific lab research.
I-FISH EFFICIENCY
First interest for the farmer is the improvement of feed efficiency:
Seabass 1st experiment: comparing the 46/12 and 45/20, we see that a reasonable different feeding (-17%) almost isonergetic gave same growth and reduced FCR in the same ratio ; so fish were given 20% less protein but fish grow the same speed and retain the same amount of protein : increased protein efficiency.
Seabream 2nd experiment: In this case the fish feed on its own appetite and the improvement was
still greater.
FCR reduced by 35% and growth increased by 3%.
Seabass : On smaller seabass, a comparison 46/12 – 45/15 shows that with a - 11% controlled feeding
gave a decrease in 17% FCR and improved growth by 3%. Here the 45/15 for these size of fish
seems suitable and very interesting for growth. This growth increase is more obvious when the fish
is bigger (70–190 g) (+ 12%) :
Here we have a relative increase in protein given to fishes due to their decreased need due to weight.
The figure in middle shows a remarkable growth take off at beginning of spring which is a criteria for very good fish condition.
It is noteworthy that mortality is decreased with this diet.
Seabream small fish (7–90)
It is interesting to see that here 45/15 gave the best result in FCR - SGR. We can observe here once again that these small fishes still need rather high protein intake.
II - FLESH QUALITY
The second fear of the farmer toward the use of high energy feed was the risk for fatty fish in general. We will focus on one parameter of flesh quality : fat %l in filet (peeled and trimed).
We compared different feeds in same farm and then different farms using same feeds.
We see that fat in fillet variation is sensible but no significant between two different feeds in one farm (feeding was reasonable and isoenergetic). But the same feed can give very different fishes according to farm strategy and situation.
Fat in filet vary of course according to weight, but also season and age :
site D = fishes of 2 years - site C = 18 months.
Seabream was sampled at a bigger weight but seems to be more fatty in flesh compared to seabass.
The same remark toward sites and strategy and strategy can be seen.
Anyways, compared to consumed salmonids flesh, we can say marines fish are still leaner (for 2
years fish) but at the same weight, it is very close = lean fish.
So provided that feed is used correctly we have shown that high energy feed (45/20 and 45/15) doesn't give more fatty flesh in production farm conditions.
We have always to remind that feed quality is responsible for only a part of results : the man who feeds the fish has 80% of the variation in his hand!…
III - GUT OUT LOSS
The third fear was fish quality as regard the carcass yield or gut out loss.
Once again there is less variation due to feed than due to farm site and higher energy feed doesn't
give bigger belly.
Gut out variation is less sensible than fat in filet variation.
Last point is decreased discharge due to higher energy feed. We have to keep in mind that criteria like digestibility is essential to discharge : a “little” variation of ADC rise in double discharge of suspended solids.
On the assumption of the black box farm concept, you won't be surprised, due to feed efficiency, that you can reach a decrease in nitrogen discharge by 31%. Result achieved after analysis of fish flesh, fish production, feed analysis, feed consumption.
60% phosphorus discharge reduction can be achieved by raw material selection; less discharge mean healthy fish and healthy environment.
Notes
FCR : Feed Conversion Rate (Kg feed/kg fish growth).
SFR : Specific Feed Rate (% of body weight/day)
SGR : Specific Growth rate (% Body weight/day)
A. M. ASUERO
EWOS SA, Spain
INTRODUCTION
The economical performance of a given farm depends largely on the production cost, since market
price influence is often out of the scope of the farmer in the short term.
We will first review the economical factors that a farm's economy in order to see
the relative importance of the feed and feeding among all. Later we will focus on these items and the
ways they might be monitored and optimised.
The optimisation of production depends, besides farm design and location, upon two factors strongly related with the feed and feeding technique employed, Total production achieved and variable cost per unit
TOTAL FISH PRODUCTION
Total production depends, in its turn upon four factors;
a) The amount of water, oxygen available, and space.
These elements determine the stock of fish, since if this figure is too big, fish may suffer stress and
their growth be reduced. In consequence, maximal production can only be obtained with the so-called
optimal stock.
Ideally, the optimal load should be maintained constantly, which means adding juvenile fish and
silling fish throughout the year
b) The duration of the growth cycle.
Growth determines the turnover of stock per year and the attainable output. If a cycle is reduced by, 15% (which is not particularly difficult) there will automatically be a 15% increase in production, without increasing fixed costs.
The duration of the cycle will be influenced by the site characteristics and the husbandry : water temperature, diet, feed efficiency, fish health and grading.
c) The mortality or system losses that reduce the total production.
d) The entry of juvenile fish.
The possibility of adding juvenile fish continuously throughout the year makes it possible for there to keep constant stock and sales. It is also important to determine the number of juvenile fish required to compensate for mortality. However, in some cases this might not justified, due to acute seasonal variation of water temperatures, which makes stocking useless during the cold period.
THE VARIABLE COST OF PRODUCTION
The variable cost per unit may be defined as the repercussion of the variable costs per kg of fish. It depends on the amount of fish kg produced / sold and is expressed in monetary units / kg. Its value will be the sum of the following factors:
a) Feed cost (monetary units) per kg of fish produced.
This value is obtained by multiplying feed price by its FCR (feed kg necessary to obtain 1 kg of fish).
For example, of feed price has a mean value of 900 US dollars / TN and a FCR of 2 kg of feed / 1
kg of fish, feed repercussion will be 1800 US dollar / TN of fish.
This calculation may be more accurate if mortality is taken into account, thus if in the previous
example there is a survival rate of 85% the net cost will be 2120 US dollars / TN, the result of dividing
FCR / survival rate.
Feed cost × FCR / Survival rate % = Net feed cost per kg.
FCR is normally calculated from rearing data that imply survival because only the weight of saleable fish is usually considered at the end of a cycle.
b) Cost of juvenile fish per kg of net fish.
In this cost, losses should be also considered. For instance, if a juvenile fish costs 0,5 US dollars, there is an 75% survival in the cycle and the fish weight when sold is 33.3 gr. (3 specimens per kg) the juvenile fish cost is 2 US dollars/ kg. Calculations are as above.
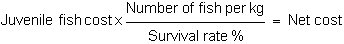
Thus one can see the advantage of selling larger fish, as the juvenile fish is, at present the greatest variable cost in production.
c) Transport and packing costs.
Although they are not proper production costs, the expenses of packing and transport are also variable costs.
d) Other costs.
Other costs may sometimes be considered as variables. For instance, the cost of treatment with drugs, pumping costs, labor costs if there are any, the cost of oxygen, insurance and replacement of some assets such as nets etc.
In practice, the two main items that determine the variable production cost are juvenile fish and feed, the mortality rate being included in both cases. The cost of permanent labor and the cost of t he initial outlay depend more on the system of farming and, therefore, vary from case to case.
FEED CHARACTERISTICS
The feed choice is a key issue when managing a farm, however it is also a very difficult one. My advice here would be to opt for a feed brand which provides reliability with field data of performance and to monitor closely the performance of this feed in order to see if the reality matches with its expectancies.
The check list for feed quality is shown in exhibit 2
A good feed shall have a balanced composition, in terms of protein and fat. The species that we are dealing with are unlike salmonids which are capable to digest/store high amounts of fat.
Bass is a white flesh true carnivorous, so high protein diet should be used, above 49%, while fat shall
not exceed 17%. While Bream is a semi-omnivorous so the diet has to be moderate in energy , 45–
47% protein 10–14% fat and type of feed has a great impact on the performance.
However, for practical reasons, it might be wise to have the same feed for both species to avoid the
complications and the high stock that feeding different diets could create. This diet could be made
meeting the common requirements of both species.
When comparing two feed remember that moisture, ash and fiber do not provide any value, and the tow later are detrimental.
The second concern comes with digestibility, feeds with abundant vegetable protein and second grade fish meals have a poer digestibility, therefore a low efficiency and greater pollution.
The energy level of a balanced diet comes by measuring the portion and fat content, as higher in energy as lower FCR expected, provided the same digestibility.
On the other hand, a tentative feed with best digestibility, high in energy and extruded (like in salmonids) would be difficult to handle since these species have an enormous appetite and overfeeding could result in sudden deaths or liver damages.
We encourage the farmers to make trials, with restricted feeding at low scale, to monitor performance and see feed differences, even if it looks laborious.
FEEDING IN HATCHERIES
We have selected the hatcheries apart from the general feeding considerations since the conditions
are significantly different.
In a hatchery the sale price of a juvenile reach 0,5 USD per piece and the feed cost per fish is close of
1% of it. Therefore the importance is the quality much more than the price.
However the quality of the feed must be monitored, otherwise you might pay 2–5 times more for a feed,
which includes just cosmetics, and obtain same results.
The feed is not always the limiting factor for performance, other items could restrict the performance
of the feed and two feeds of different quality perform equally. Our advice in this matter is double:
Choose a feed according with your husbandry level.
Improve the husbandry and other factors and rechec the feeds accordingly.
The hatchery management has possibilities to make quick and reliable trials, and this advange should be used. The cost of putting aside 4–6 tanks for checking other hatchery feeds might be the best investment, and it would also allow to test feeding techniques and savings on artemia by earlier or quicker weaning.
Be aware that hatchery technicians are not always interested on testing other possibilities options or new husbandry techniques, since it creates extra work and they might not enjoy the benefits. Its, obviously, the Manager's task to enhance testing and motivate the technician accordingly.
A hatchery feed, among other features, has to be:
Very reliable and quality consistent first, since the weaning process is generally suited to specific feed.
High in energy and well balanced, since the metabolism is very quick and growth demands fast
and complete feed transfer into flesh.
If these requirements are not met mortality and cannibalism occur, remember that the cost of 10 fish
is equivalent of 1 Kg feed.
Particle size and physical quality are very important at weaning, specially when automatic feeders are used. Particles shall be homogeneous within the given range, and dosage should flow regularly to avoid clumping due to moisture arising from the tank.
The feed shall be of very high digestibility, (low ash and fiber). This helps to avoid extra material in the tank which would promote bacterial culture.
These are some examples of feed quality, the list could be enlarged by the your hatchery technician.
Another aspect worth to mention would be feeding, we have seen in some cases that when feed trials
are done, the result might be erroneous because the feeding amount has not been adapted to the different
feed energy.
The feeding amount is something which has to be discussed between the feed company and the farmer
and should at least be followed during the trial.
FEEDING IN ONGROWING
Feeding ongrowing fish is a key issue in any farm's profitability, however it might not be the limiting factor. The first recommendation, when looking to improve feed performance, would be to find out what is the limiting factor on the farm's performance, otherwise the efforts directed to improve the performance by the feed might result useless.
These are some suggestions:
Stock, high stoking rates would diminish the performance of any feed.
Oxygen, low oxygen levels or sudden changes, would decrease digestibility
Temperature, and salinity. Or sudden changes which create stress to fish
Any other form of stress.
The factors related with the feed are two:
Feed quality, as the capacity to generate growth. Energy, balance and digestibility
Feeding system or husbandry. This is of particular importance since Bass and Bream, at certain temperatures, tend to feed more that needed and ad libitum feeding results in poor FCR and other problems.
We feel that this issued needs to be elaborated further by the scientific community.
From our own experience on this matter and we are more and more convince that feeding has to be limited if improvement in FCR has to be forthcoming.
If I was asked what would be the most important idea that I would like to transmit to this audience I would insist on the economical importance of feeding these species and improving the systems to monitor performance.
SUMMARY
The feed and the feeding system is the most important item of those that influence cost of production. The feed quality is important but often is more important is to be able to monitor the real performance of the feed
At a hatchery the feed performance would focus on consistency in quality and securing a good survival.
At ongrowing farms the feed has to compromise quality and price to porvide a competitive option
to the farmer.
The feed might not always be the limiting factor in performance, therefore a two way effort has to
be made in order to continuously improve the husbandry and using the most efficient feed for that
husbandry.
Sustainable improvements in husbandry, together with a genetic upgrading plan, would allow nutritionist
a constant development on diets resulting in better efficiency.
This combination would resemble what other farmed species have experienced, terrestrial of fish.
The farmers role in this development is to endeavour to a husbandry improvement program and
secure records keeping of performance, which would allow further conclusions.
The scientifical community shall look into the specific problems of this business and research on
priority needs, even if it looks rather basic.
Bass and bream require a feeding plan that restrict feeding in some cases, is important to devote more attention to this matter in order to find out the most economical feeding table and the most economical feed.
FACTORS AFFECTING THE SHORT TERM PROFITABILITY OF A FARM, feed related are underlined
1 TOTAL PRODUCTION
- Water, space and oxygen limitations.
- Optimization of the stocking density in cages and ponds.
- Constant supply of juvenile fish throughout the year.
- Constant sale of fish.
- Mortality.
- Duration of growth cycle.
- Temperature
- Diet: time per day, efficiency etc.
- Efficiency of feed in promoting growth.
- Fish classification.
- Fish health, avoidance of stress.
2 VARIABLE COST PER UNIT.
- Feed repercussion per Kg. fish.
- Juvenile fish cost, taking mortality into account.
- Transport and packaging costs.
QUALITY OF THE FEED, CHECK LIST.
1 PHYSICAL QUALITY
- Sizes available and size distribution, length and diameter.
- Dust content, particle contamination
- Packing and labeling
2 CHEMICAL QUALITY
- Protein/fat ratio
- Non digestible matters, ash, fiber and moisture.
- Digestible energy, energy concentration.
- Vitamins and expire date.
3 SERVICE QUALITY
- Delivery period.
- Reliability and consistency
- Technical assistance, production, veterinary.
- Expected performance, FCR, Growth, trails, experience.
- Innovation.
Ewos Aquastart Marine
Weaning diet for Bream & Bass
Contents :
| Protein | 52 % | |
| Fat | 14 % | |
| Fiber | 1 % | |
| Ash | 9,5 % | |
| Moisture | 10 % |
Particle size :
| AM 0 | 150–300 microns | |
| AM 1 | 300–600 microns | |
| AM 2 | 600–1000 microns |
Package : 10 kg. net weight Bins
Characteristics :
The diet Ewos Aquastart Marine contains a selected mix of marine proteins of the highest digestibility. This allows an optimun growth rate and diminishes the mortality of the weaning stage.
Its composition regarding fatty acid PUFA type W-3 guarantees the minimum requirements of these essential ingredients.
Ewos Aquastart Marine has been formulated with most stable vitamins and attractants, like Betaine, to enhance the weaning process.
With the new manufacturing process, the feed flows more easily on automatic feeders, since the moist absorption has been diminished.
Ewos Aquastart Marine
Weaning diet for Bream & Bass
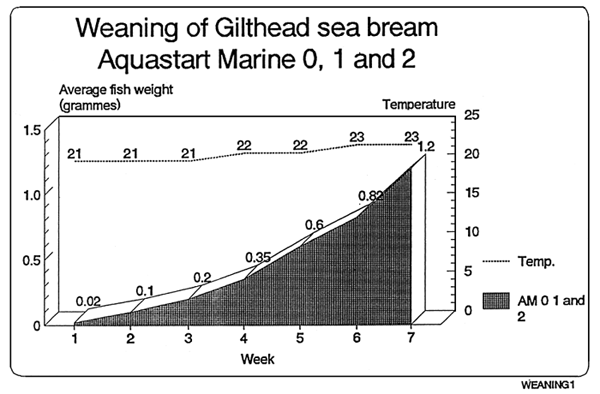
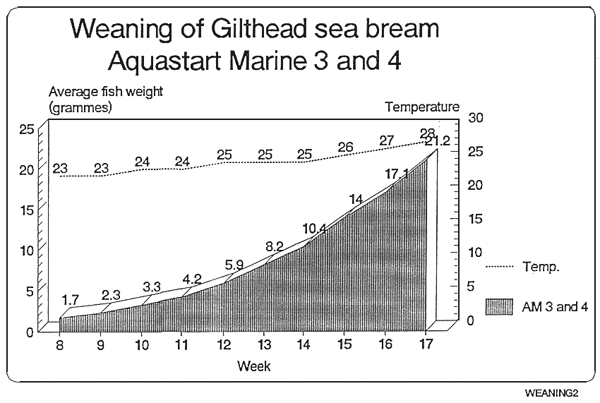
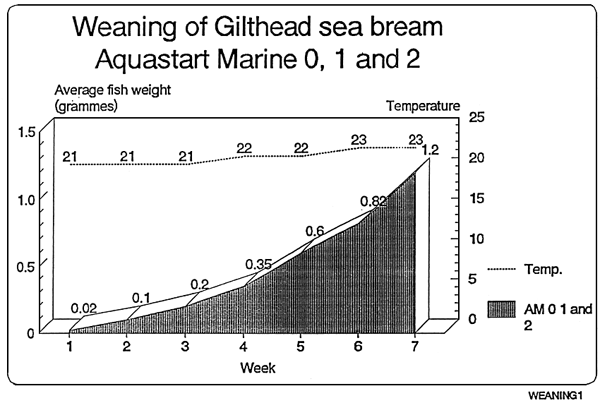
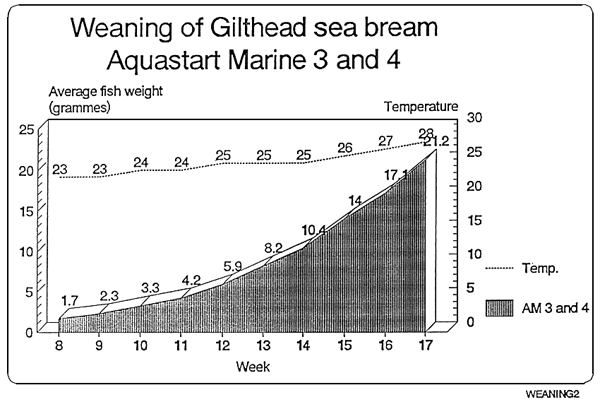
Feeding chart for sea-bream
The following values are orientative since this largely depends on the strain of the fish.
| Feed | Sea-bream | Daily % |
|---|---|---|
| Size | Size | on fresh weight |
| AM 0 | 0,01–0,07g. | 10% |
| AM 1 | 0,07–0,35g. | 8% |
| AM 2 | 0,35–0,60g. | 7% |
| AM 2 | 0,60–1,20g. | 6% |
* Ewos recommends the following:
The product shall be adapted to the current husbandry used, slowly and gradually. It might be the case that less amount of feed in needed than with other products, since Ewos Aquastart Marine has a higher energy content
In case of doubt it is advisable to overfeed rather than underfeed, in the former case a closer look at the health status of the tank shall be taken, whereas on the latter cannibalism might occur.
When changing from one size to a larger one, it must be done after a period mixing both sizes.
In case it is needed, feel free to contact directly EWOS technicians for more information.
FEEDING IN ONGROWING
feeding ongrowing fish is a key issue in any farm's profitability
What is the limiting factor? Some suggestions to check:
- Stock, high stocking rates would diminish the performance of any need
- Oxygen, low oxygen levels, or sudden changes
- Temperature, and salinity, or sudden changes.
- Any other form of stress or diseases.
The factors related with the feed are two:
- Feed quality, as the capacity to generate growth.
- Feeding system or husbandry.
Feeding has to be limited of improvement in FCR is to be forthcoming.
EWOS Bass & Bream
Ecological Declaration:
Diets: Juvenile. Grower & Bream Pigmented
For a Production of 1000 kg fish.
Composition in % :
| Diet | Juven. | Grower & Pigment | |
|---|---|---|---|
| Protein | 54% | 49% | |
| Phosphorus | 1,3% | 1,2% | |
| FCR | 0,9 | 2,2 | |
| Digestibility | 90% | 85% | |
| * Fat digest, | 88% | 88% | |
| * Prot.digest | 80% | 80% | |
| * CH.digest. | 75% | 75% | |
| *Phosph.dig. | 45% | 40% | |
| * Energy dig. | 17,4 Mj | 16,5 MJ | |
Environmental impact, breakdown in Kg -
| Diet | Juvenile | Grower & Pigment |
|---|---|---|
| Prod. solids (Kg) | 90 | 330 |
| Phosph. via feed | 11,7 | 26,4 |
| Phosph. in fish | 5 | 5 |
| Phosph. in faeces | 6,4 | 15,84 |
| Phosph. in water | 0,26 | 5,56 |
| Nitrog. via feed | 77,8 | 172 |
| Nitrog. in fish | 30 | 30 |
| Nitrog. in faeces | 15,5 | 34,5 |
| Nitrog. in water | 32,2 | 108 |
EWOS Bass & Bream
Diets: Juvenile, Grower & Bream Pigmented
Composition in %
| Diet | Juvenile | Grower | Pigment |
| Protein | 54% | 49% | 49% |
| Fat | 14% | 12% | 12% |
| Fiber | 1% | 2.5% | 2.5% |
| Ash | 9% | 12% | 12% |
| Moist | 10% | 10% | 10% |
Energy
| Digest | 17,4Mj | 16,5Mj | 16,5Mj |
Sizes:
| Juvenile: | Crumbs 2 | 0,6–1,0 mm |
| Crumbs 3 | 1,0–1,4 mm | |
| Crumbs 4 | 1,4–2,0 mm |
Grower: pellets 2 mm, 3 mm, 4'5 mm & 7 mm
Bream Pigmented: pellets 4'5 mm & 7 mm
Packing:
Crumbs in 25 Kg. for grower & pigment 25 Kg, or big-bags
Pigmentation:
* The Bream pigmented diet is formulated to develop the colour of the wild fish. The usage has to last for 4–6 weeks in summer temperatures and longer with colder waters; the colour will depend on the amount of feed provided so it is important to check the colour by the farmer.
The flesh does not develop any colour.
Broostock feed:
* EWOS has a well tested Brookstock diet available, if interested contact our sales representative.
J.M. Fernandez
EWOS SA, Spain
1. INTRODUCTION.
- Ewos Aquaculture is a multinational group with a production around 150,000 tons/year. of fish/feeds.
- Ewos Spain has the responsability of the mediterranean area and is focusing on marine species of the Mediterranean Sea as Sea bream and Sea bass, including Turbot.
2. AIMS FOR AN R & D PLAN.
To open Ewos Aquaculture to the Mediterranean market.
To obtain diets more specific and profitable and adapted to mediterranean culture conditions, avoiding to apply the technology used in other fish cultures like salmonids.
3. 1990, SET UP OF THE WORKING GROUP.
- In this research group is created to carry through the R & D plan.
- An agreement is signed with Spanish Oceanographic Institute to carry out the trials.
4. PERIOD 1991–1993
Budget: 50 million ESP.
Targets:
Weaning diets.
- To create a new commercial diet.
Growing diets in Bream and Bass.
- New diet under these specifications FCR=2, SGR=2.
- Obtention of a feeding chart.
- Comparison between different manufactoring systems. (Extrusion, pelletation, HTST).
- Obtention of pigmented bream diets.
Growing diets in Turbot.
Obtention of a dry diet replacing therefore semi-moist diet used in the market.
Result comparisons between dry diets of different energy levels.
Utilization of automatic feeders.
All experiences were carried out under same criteria of control and stadistics point of view.
All trials done in the researche Centres were afterwards developped in commercial fish farms.
SUMMARY ON FINAL RESULTS 91–93
Weaning diets.
- A new diet was obtained with a better performance and a cost closer to the growing feeds.
Growing diets to Bream and Bass.
The best results were obtained with diets with 48% protein content and 12% of fat content (Medtabolizable energy aroun 15MJ/Kg.).
A feeding chart for seabream was made and a control of feeding ratio is recomended. It is necessary to avoid the overfeeding.
There were no significant differencies with different systems of feed manufactoring. (Pellet and extrusion) as fr as performance in trials is concerned.
A finished bream diet was obtained.
Gorwing diet for turbot.
A dry feed as an alternative to semimoist feed was produced
The extruded diet (54% protein/17% fat) gave better results than the pellet diet, especially in turbot weight from 400 grs.
5- NEW PERIOD 1993–1995.
Budget: 75 million ESP.
Targets:
New diets with better performance and reducing the growing period.
Marking feeding chart for seabass and turbot.
New specific diets for different farming systems.
- In land ponds.
- Cages.
- Land based ponds.
Improving feed digestion and reduction on pollutions effects.
Obtention of new finished diets.
Development of diets for new species. (Sole, red sea bream, and so on).
6. - SUMMARY.
- The specific diets will enable to increase farming efficiency and therefore profitability.
- The collaboration and ideas exchange between the technicians and managers of mediterranean fish farms are vital for future development of mediterranean aquaculture.
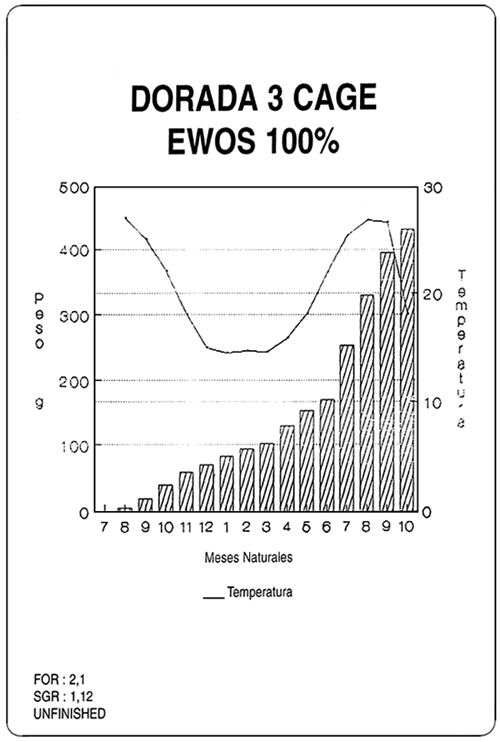
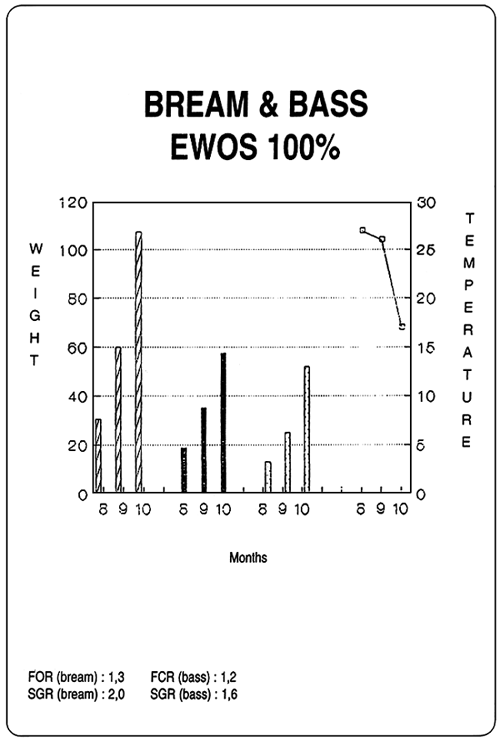
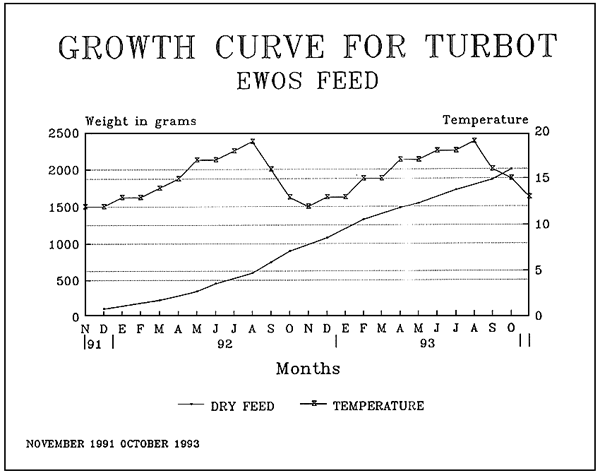
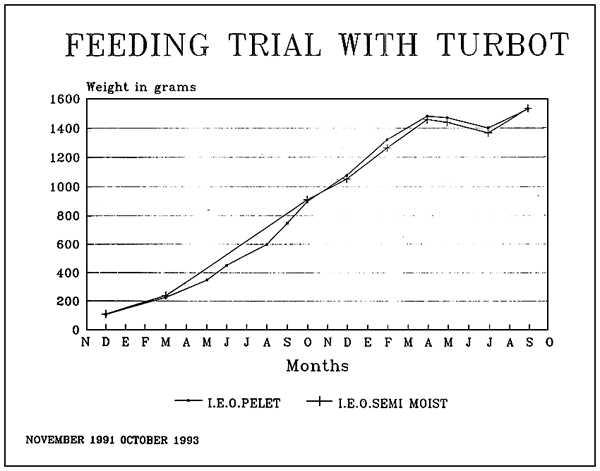
| Diets for sea-bass and sea-bream | ||||||||||||
| APRIL-1993 | ||||||||||||
| Feeding table for sea-bream and sea-bass. | ||||||||||||
| Daily percentage of feeding on fish weight | ||||||||||||
| Normal farming conditions - SEA-BREAM | ||||||||||||
| Temp. | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | ||
| Mean weigh | Size of | |||||||||||
| of fish (g.) | feed (mm) | |||||||||||
| For fish under 1 g. see feeding table of Ewos Aquastart Marine | ||||||||||||
| 1–5 | Crumbles 3 | 3,30 | 3,50 | 4,50 | 4,70 | 5,00 | 5,50 | 5,70 | 5,80 | 5,90 | 6,00 | |
| 4–14 | Crumbles 4 | 2,48 | 2,63 | 3,38 | 3,53 | 3,75 | 4,13 | 4,28 | 4,35 | 4,43 | 4,50 | |
| 14–20 | Pellet 2mm | 1,50 | 1,70 | 1,90 | 2,00 | 2,10 | 2,80 | 3,20 | 3,50 | 4,00 | 4,10 | |
| 20–40 | Pellet 2mm | 1,30 | 1,40 | 1,50 | 1,70 | 2,00 | 2,50 | 3,00 | 3,30 | 3,50 | 3,60 | |
| 40–60 | Pellet 3mm | 1,00 | 1,10 | 1,20 | 1,40 | 1,50 | 2,00 | 2,30 | 2,50 | 3,00 | 3,10 | |
| 60–100 | Pellet 3mm | 0,80 | 0,90 | 1,00 | 1,50 | 1,70 | 1,90 | 2,10 | 2,30 | 2,50 | 2,70 | |
| 100–200 | Pellet 4.5mm | 0,70 | 0,80 | 0,90 | 1,00 | 1,20 | 1,40 | 1,70 | 1,80 | 2,00 | 2,20 | |
| 200–300 | Pellet 4.5mm | 0,60 | 0,70 | 0,80 | 0,90 | 1,10 | 1,30 | 1,40 | 1,50 | 1,50 | 1,50 | |
| 300–500 | Pellet 4.5mm | 0,50 | 0,60 | 0,70 | 0,80 | 0,90 | 1,10 | 1,30 | 1,40 | 1,40 | 1,40 | |
| 500–1000 | Pellet 7mm | 0,40 | 0,50 | 0,60 | 0,70 | 0,90 | 1,00 | 1,00 | 1,10 | 1,10 | 1,10 | |
| Optimum farming conditions/accelerated growth -SEA-BREAM | ||||||||||||
| Temp. | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | ||
| Mean weigh | Size of | |||||||||||
| of fish (g.) | feed (mm) | |||||||||||
| For fish under 1 g. see feeding table of Ewos Aquastart Marine | ||||||||||||
| 1–5 | Crumbles 3 | 3,30 | 3,50 | 4,50 | 4,70 | 5,00 | 5,50 | 5,70 | 5,80 | 5,90 | 6,00 | |
| 4–14 | Crumbles 4 | 2,48 | 2,63 | 3,38 | 3,53 | 3,75 | 4,13 | 4,28 | 4,35 | 4,43 | 4,50 | |
| 14–20 | Pellet 2mm | 1,50 | 1,70 | 1,90 | 2,40 | 2,90 | 3,50 | 4,00 | 4,50 | 4,50 | 4,60 | |
| 20–40 | Pellet 2mm | 1,30 | 1,40 | 1,50 | 2,00 | 2,30 | 2,60 | 3,40 | 4,00 | 4,00 | 4,00 | |
| 40–60 | Pellet 3mm | 1,00 | 1,10 | 1,20 | 1,40 | 1,80 | 2,20 | 2,60 | 3,50 | 3,50 | 3,50 | |
| 60–100 | Pellet 3mm | 0,80 | 0,90 | 1,00 | 1,50 | 1,70 | 2,00 | 2,50 | 3,00 | 3,00 | 3,00 | |
| 100–200 | Pellet 4.5mm | 0,70 | 0,80 | 0,90 | 1,10 | 1,50 | 1,80 | 2,00 | 2,60 | 2,60 | 2,60 | |
| 200–300 | Pellet 4.5mm | 0,60 | 0,70 | 0,80 | 1,00 | 1,30 | 1,50 | 1,80 | 2,20 | 2,20 | 2,20 | |
| 300–500 | Pellet 4.5mm | 0,50 | 0,60 | 0,70 | 0,90 | 1,21 | 1,30 | 1,50 | 1,80 | 1,80 | 1,80 | |
| 500–1000 | Pellet 7mm | 0,40 | 0,50 | 0,60 | 0,80 | 1,00 | 1,20 | 1,30 | 1,50 | 1,50 | 1,50 | |
| Notes | ||||||||||||
| • FOR SEA-BASS INCREASE FEEDING 10% | ||||||||||||
| • Fish have better growth and conversion with increasing photoperiod | ||||||||||||
| • In case of oxygen depletion, decrease feeding at high temperatures. | ||||||||||||
P. Dhert
Introduction
The expertise developed at British and French research institutes led to the set-up of the first commercial
hatcheries of the European seabass Dicentrarchus labrax, the gilthead seabream Sparus aurata
and the turbot Scophthalmus maximus in the early eighties.
In this review we want to give a short overview of what we consider to have been, and to some extent
might still be, important bottlenecks in the successful larviculture of marine fish.
Nutritional Requirements
Without any doubt, the most significant progress achieved during the last decade has been the result of improved nutrition, more particularly through the manipulation of the fatty acid profile of the live preys Brachionus and Artemia (reviews in Sorgeloos and Léter, 1992; Lavens et al., 1993). We have been misled by the most widely used food item, the brine shrimp Artemia of which today more than 2000 metric tonnes of cysts are marketed annually for feeding in fish and shellfish hatcheries. As the studies of Watanabe et al. (1993) and léger et al. (1995) revealed that the presence of the fatty acid 20:5 (n-3) (eicosapentaenoic acid or EPA) determined the suitability of a given Artemia source for marine fish larvae, all emphasis was on increasing the EPA levels in the live preys Branchionus and Artemia by using algae, or, emulsified and particulate enrichment products (review in léger et al., 1986).
Nonetheless, more attention should have been paid to the levels of 22 : 6 (n-3) (docosahexaenoic acid or DHA) when evaluating the results of feeding tests with different Artemia preparations for the larvae of the European seabass (Lisac et al., 1986) : good survival indeed appeared to be correlated with high EPA levels in the diet; however, best growth was achieved in the diets with the highest DHA levels. Recent studies with various species have revealed the importance of DHA and more particularly the requirement for high-DHA/EPA ratios in promoting growth, stress resistance and pigmentation (Lavens et al., 1993; Kraul et al., 1992; Devresse et al., 1992, and Mourente et al., 1993). whereas in the past satisfactory results were achieved with DHA/EPA ratios of less than 1 all emphasis is now to reach levels of 4 and higher in the live preys Brachionus and Artemai in the evaluation of diet performance (Dhert et al., 1993b). As it was pointed out by Sargent et al. (1992) that neural tissues contain elevated concentrations of DHA it is not unlikely that the DHA-deficient live preys that have been offered sofar to marine fish larvae resulted in poor performing animals with regard to visual perception of the prey and other behavioral aspects.
The requirements in the larval diet for (n-3) HUFAs might also vary as a function of larval quality. This could be illustrated with turbot larvae at first feeding. Early in the turbot reproductive period, when egg and larval quality are generally at their best, the requirement for DHA enrichment in the rotifer diet is reduced to two days only, whereas late in he season, when poor-quality larvae prevail, DHA needs are high (Dhert et al., 1993 a). Furthermore Harel et al. (1993) documented a clear effect of the fatty acid composition of the material diet on the egg quality of Sparus aurata. Last year we documented that in their early larval development, turbot can turn black as a result of stressed conditions, e.g. unsuitable light conditions or elevated larval densities (Lavens et al., 1993). Recovery to a normal color appears to be function of the (n-3) HUFA composition of the diet, i.e. slow when offered a coconut-enriched Brachionus, fast when feeding EPA and DHA enriched diets.
It is clear that in the field of larval nutrition most attention sofar has been paid to the qualitative and quantitative (n-3) HUFA requirements; further work is required to verify ratios of n-3/n-6/n-9 fatty acids, and especially to evaluate the effects of other lipid classes, not the least the phospholipids which might alter the requirements for (n-3) HUFAs. Aside from the lipids it is clear that many other dietary components deserve more attention in future larviculture nutrition research, e.g. different vitamins (recent work by Merchie et al., 1993 illustrates significant effects in growth and/or stress resistance with vitamin C in different species0, free amino acids, pigments, etc.
In our opinion the challenge remains to analytically and biologically unravel those components present in wild copepods and responsible for their superb dietary value for marine fish, eventually allowing their incorporation in the practical live food chain Brachionus and/or Artemia. Similarly, the magic use of “green water” in the commercial rearing of most marine fish species requires further study various hypotheses are worth pursuing, e.g. as a source of micro-nutrients, source of immunostimulants, water quality conditioner, microbiological conditioner (probiont, see further).
Microbial Control
An area that urgently deserves more attention is the microbial flora of marine fish hatcheries. With the upscaling and expansion of commercial fish larviculture, hatcheries have been plagued by increased incidence of microbial diseases, often claimed to be caused by Vibrio spp. (several comm. pers. With marine fish hatchery operators in the Mediterranean). Similar to practices in marine shrimp hatcheries, the indiscriminate use of antibiotics in prophylactic treatment of the fish tanks resulted in the development of resistant strains and the need to switch to other antibiotics, a practice which is doomed to fail (Brown, 1989).
Thanks to support from the European Community FAR-office, an extensive study has been initiated to get a better fundamental understanding of the microbial environment of two marine fish hatcheries in the Mediterranean. An extensive sampling campaign was performed during the first 30 days of the larval rearing of seabass Dicentrarchus labrax and seabream Sparus aurata; i.e. the microbial diversity and their quantitative presence were investigated, as well as the relationship between the microflora of the culture water, the live food, and the subsequent colonisation of the larval intestine during development. The microbial diversity appeared to be enormous (over 1200 bacterial strains have been characterised) and varied from season to season as well as from hatchery to hatchery.
In one hatchery there was a clear succession of bacterial species in function of time and live food administered; a fish mortality outbreak could be correlated with an increase in Vibrio anguillarum during rotifier feeding. In the other hatchery the diversity of bacterial species, including non-Vibrios was much higher and no larval mortalities were recorded.
Several trials to challenge 35 to 40-day old seabass larvae with V. anguillarum reference strains and isolates from the sampling campaign failed to induce mortality. It remains unclear, at least with sea-bass larvae, if one is dealing with obligate or opportunistic pathogens, or if the V. anguillarum strains isolated in this study belonged to a serotype pathogenic for young larvae and not for older juveniles.
In similar challenge tests with turbot Scophthalmus maximus larvae (Chair et al. 1993) could prove pathogenicity with some of the same V. anguillarum strains.
Quatitative figures reveal the presence of 100–10,000 bacteria per rotifier and per brine shirmp nauplius. The bacterial numbers found in the fish larvae increase with age and type of feeding from 1,000 to 100,000 per fish larva. It is interesting to note that the numbers found on a Vibrio specific TCBS - medium were always 10 to 100-fold lower than the total counts on a marine agar culture medium, except in cases of mortality outbreaks when the values were similar. The fact that the composition of the microflora in the intestine of healthy fish is not an exact copy of the microflora found in the culture water nor the live food, might be an indication for selective colonisation of the gut or the existence of so called probiotic bacteria.
The major surprise of this microbiological study is the important input of bacteria (and potentially fish pathogens0 via the live food chain. One should consider new measures to reduce bacterial loads as well as to selectively manipulate the microflora both in the live feeds produced in the hatchery and in the culture water prior to stocking of the fish larvae. Application of new disinfecting procedures for Artemia hatching and enrichment appear to be promising (Dehasque et al.,1993). Introduction of Branchionus production tanks with selected probionts results in improved rotifier culture outputs and eventually reduced bacterial contamination in the turbot larvae (Bogaert et al., 1993; Gatesoupe, 1991). Selected bacterial inoculation of the culture tank prior to stocking with the larvae would not only reduce the chances that opportunistic bacteria become dominant, but eventually have a beneficial effect on first colonization of the fish larva's gut.
In general, very strict hygienic measures should be taken in the hatcheries. Furthermore regular disinfecting and dry-out of the complete culture circuit (including all piping) in between production cycles should be implemented. In that respect new hatcheries should consider the use of modular systems rather than to have all culture units in one building.
When antibiotic treatment is justified for therapeutic reasons, one should not add drugs to the culture tank but rather apply oral biomedication using Brachionus and/or Artemia as a carrier for the antibiotics ingested from an emulsified or particulate preparation. Doses ranging from 20 to 100 ppm sulfadrugs can be incroporated in seabass respectively turbot larval tissue within less than 4 h after feeding Artemia metanauplii boosted with the antibiotics (Chair et al., 1991; 1993). The therapeutic efficacy of this oral delivery system has been documented by Chair et al. (1993) and Dhert et al. (1993) with turbot larvae challenged with a Vibrio anguillarum pathogen.
Conclusion
The intensification of hatchery activities brought about new problems not seen at an experimental scale, e.g. in relation to upscaling of live food production, washing and cleaning of live food, intensity of manual labour, etc. Sofar too limited attention has been paid to improved zootechniques which might make marine fish larviculture more predictable and more cost-effective, e.a. selection and use of new materials (f.ex. stainless-steel welded-wedge filters instead of woven filters for Artemia washing, Léger and Sorgeloos, 1992), increased automation as to reduce the so-called “human factor” often responsible for reduced performances after several months of operation, etc.
In the past decade marine fish hatchery have evolved from hit and miss ventures into profitable entreprises. In Europe, market saturation of bass, bream and even turbot fry has been reached faster than was anticipated at the World Aquaculture 1990 meeting in Halifax, Canada (Sorgeloos and Léger, 1992); i.e. fry prices have dropped to US $ 0.75 and less per larva (Stephanis and Divanach, 1993).
Although there is still room for further improvements especially with regard to cost-effectiveness, present-day hatchery technology for marine fish species has proven to be widely applicable, both in terms of geographic location as well as fish species. The list of commercially cultivated species is growing fast (e.a. various bass and bream species, turbot, Japanese flounder, fugu, milkfish, mullet) and many candidate species will hopefully soon join this list, e.g: dolphin fish mahi-mahi, various grouper species, red snapper, cod, wolffish.
REFERENCE
BOGRAERT, P, DEHASQUE, M., and SORGELOOS, P. 1993. Probiotic effects of bacteria on the growth of the rotifer Brachionus plicatilis in culture. In Book of Abstracts “World Aquaculture” “93”, p. 321. Ed. by M. Carillo, L. Dahle, J. Morales, P. Sorgeloos, N. Svennevig and J. Wyban. European. Aquaculture Society, Special Publication No. 19. Cent, 632 pages.
BROWN; J. 1989. Antibiotics: their use and abuse in aquaculture. World Aquaculture, 20(2) : 34–43.
CHAIR, M., ROMDHANE, M., DEHASQUE, M., NELIS, H., DE LEENHEER, A.P., and SORGELOOS, P. 1991. Live-food mediated drug delivery as a tool for disease treatment in larviculture. II. A case study with European seabass. In Larvi'91 - Fish & Crustacean Larviculture Symposium, pp. 412–414. Ed. BY P. Lavens, P.
SORGELOOS, E. JASPERS and R OLLEVIER. European Aquaculture Society, Special Publication No. 15, Gent. 427 pages.
CHAIR, M., DEHASQUE, M., VAN POUCKE, S., SORGELOOS, P., and NELIS, II. 1993. Livefood mediated drug delivery as a tool for disease treatment in larviculture. III. A case study with turbot (scophthalmus maximus). In Book of Abstracts 'World Auqaculture '93, p. 335 Ed. by M. Carillo, L. Dahle, J. Morales, P. Sorgeloos, N. Svennevig and J. Wyban. European Aquaculture Society, Special Publication No. 19, Gent. 632 pages.
DEHASQUE, M., DEVERSSE, B., AND SORGELOOS, P. 1993. Effective suppression of bacterial bloom during hatching and enrichment of Artemia and its applicability in fish/shrimp hatcheries. In book of abstracts “World Aquaculture'93”, p. 347 Ed. By M. Carillo, L. Dahle, J. Morales, P. Sorgeloos, N. Svennevig and J. Wyban, European Aquaculture Society, Special Publication No. 19, Gent. 632 pages.
DEVERSSE B., LÉGER, P. SORGELOOS, P. MURATA O., NASU, T., IKEDA, S., RAINDUZZO, J.R. REITAN, K. I., KJORSVIK E. AND OLSEN, Y. 1992. Improvement of flatfish pigmentation through the use of DHA enriched rotifers and Artemia. In book of Abstracts, V International Symposium of Fish Nutrition and Feeding, September 7–10, 1992, Santiago, Chile.
DHERT, P., LAVENS, P., DEHASQUE, M., and SORGELOOS, p. 1993a. Improvements in the larviculture of turbot Scopthalmus maximus; zootechnical and nutritional aspects, possibility for disease control. In proceeding workshop Turbot-culture: Problems and prospects, Torremolinos, Spain. EAS Special Publication, Gent (in press).
DHERT, P., SORGELOOS, P. and DEVRESSE, B. 1993h. Contributions towards a specific DHA enrichment in the live food Brachionus plicatilis and Artemia sp. In fish Farming Technology, pp. 109–115. Ed. by II. Reinertsen. L.A. Dahle, L. Jorgensen and K. Tvinnerim, A.A. Balkema, Rotterdam. 482 pages.
GATESOUPE F.J. 1991. THE effect of three strains of lactic bacteria on the production rate of rotifers, Brachionus plicatilis, and their dietary value for larval turbot, Scophthalmus maximus. Aquaculture, 96:335–342.
HALEL, M., TANDLER, A., and KISSIL G. WM. 1992. The kinetics of nutrient incorporation into body tissues of gilthead seabream S. aurata females and subsequent effects on egg quality. Aquacluture and Fisheries Management (in press).
KRAUL, S., NELSON, A., BRITTAIN, K., AKO, H., and OGASAWARA, A, 1992. Evaluation of live feeds for larval and postlarval Mahimahi Coryphena hippurus. Journal of the World Aquaculture Society, 23(4): 299–306.
LAVENS, P., SORGELOOS, P. DHERT, P., and DEVRESSE, B. 1993. Latest developments in larval food production. Aquaculture and Fisheries Management (in press).
LÉGER, PH, BENGTSON, D.A., SIMPSON, K.L., and SORGELOOS, P. 1986. The use and nutritional value of Artemia as a food source. Oceanogr. Mar. Biol. ann. Rev. 24:521–623.
LÉGER, PH., and SORGELOOS, P. 1992. Optimised feeding regimes in shrimp hatcheries. In Marine Shrimp Culture: Principles and Practices, pp. 225–244. Ed. by A.W. Fast and L.J. Lester, Elsevier, New-York. 862 pages.
LÉGER, P., SORGELOOS, P., MILLAMENA, O.M., and SIMPSON, K.L. 1985. International study on Artemia. XXV. Factors determining the nutritional effectiveness of Artemia: The relative impact of chlorinated hydrocarbons and essential fatty acids in San Francisco Bay and San Pablo Bay Artemia. Journal of experimental marine Biology and Ecology, 93: 71–82.
LISAC, D., FRANCEVIC, V., VÉJEMELKA, Z. BUBLE, J., LÉGER, PH., and SORGELOOS, P. 1986. International study on Artemia. XLIII. The effect of live food fatty acid content on growth and survival of sea bream (Sparus aurata) larvae. Paper presented at the conference Ichthypopathology in Aquaculture, October 21–24, Inter-University Center, Dubrovnik.
MERCHIE, G., LAVENS, P. NELIS, H.L., SORGELOOS, P. and DE LEENIHEER, A., 1993. THE effect of feeding vitamin C-entriched live food on the hatchery production of Macrobrachium rosenbergii. In Book of Abstracts “World Aquaculture '93”, P. 149. Ed. by M. Carillo, L. Dahle, J. Morales, P. Sorgeloos, N. Svennevig and J. Wyban. European Aquaculture Society, Special Publication No. 19, Gent. 632 pages.
MOURENTE G., RODRIGUEZ, A., TOCHER, D.R., and SARGENT, J.R. 1993. Effects of dietary docosahexaenoic acid (DHA; 22:6 n-3) on lipid and fatty acid composition and growth in gilthead sea bream (Sparus aurata L.) larvae during first feeding. Aquaculture, 112: 79–98.
SARGENT, J. R. BELL. J.G. BELL, M.V., HENDERSON, R. J. and TOCHER, D.R. 1992. The metabolism of phospholipids and polyunsaturated fatty acids in fish. In Proc. of Europ. Soc. Comp. Physiol. & Biochem. Experimental aspects of Aquaculture, Antibes. Oct. 1991. in press.
SORGELOOS, P., and LÉGER, P. 1992. Improved larviculture outputs of marine fish. shrimp and prawn. Journal of the World Aquaculture Society, 23 (4): 251–264.
STEPHANIS, J., and DIVANACH, P. 1993. Farming of mediterranean finfish species, present status and potentials. In Book of Abstracts “World Aquaculture'93” , pp. 290–291. Ed. by M. Carillo, Dahle, J. Morales, p. Sorgeloos, N. Svennevig and J. Wyban. European Aquaculture Society, Special Publication No. 19, Gent. 632 pages.
WATANABE, T. KITAJIMA, C., and FUJITA, S. 1983. Nutritional values of live organisms used in japan for mass propagation of fish: a review, Aquaculture, 34:115–143.