J. Régnière
Jacques Régnière is with Natural Resources Canada, Canadian Forest Service, Sainte Foy Station, Quebec, Canada.
Knowledge about an insect species’ developmental responses to key climatic factors, especially temperature, is used to predict the species’ potential geographic range and performance under climate change.
| The gypsy moth, Lymantria dispar: models of the seasonality of this introduced pest in North America predict that it will expand further north and west into Canada, where it could threaten considerable hardwood forest resources |
 |
J.H. Ghent/US Forest Service/Bugwood.org |
Much evidence is accumulating that insect distributions are changing in unprecedented ways. Alterations in the Earth’s climate are providing mobile insect species with increasingly hospitable habitats, and increasing global commerce is expanding opportunities for mobile species to colonize new habitats.
This article describes potential impacts of climate change on forest insect species and an approach for predicting their distributions based on their known physiological responses to specific weather factors. The modelling is based primarily on developmental responses, as these determine climates under which an insect can achieve a stable, adaptive seasonality. Models can also take into account other weather influences such as cold tolerance. Three examples are given from North America: the native spruce budworm (Choristoneura fumiferana); the introduced, invasive gypsy moth (Lymantria dispar); and the native mountain pine beetle (Dendroctonus ponderosae).
These studies predict that the distribution of most insect species will shift towards the poles and to higher elevations with predicted climate change, and that temperate regions will bear the main burden of these shifts. Distribution shifts may be good or bad, depending on the species and the point of view; but the models suggest that a warmer world is not necessarily a world with more pests.
Insects constitute the most diverse form of animal life in terrestrial ecosystems. Most species are innocuous and essential components of natural ecosystems. Because they are cold-blooded, the rates of key physiological processes in their life cycles are determined by environmental conditions, especially temperature and precipitation. In general they have short generation times, high fecundity and high mobility (either through their own faculties or aided by wind, animals and humans). The effects of climate change on forest insects (reviewed in Moore and Allard, 2008) must be considered in the context of increasing international trade and changing land-use patterns.
The fossil record suggests that previous episodes of rapid global warming led to increased levels of insect herbivory (Currano et al., 2008). Similarly, insect herbivory levels are currently increasing (DeLucia et al., 2008), for example in the birch forests of northern Europe (Wolf, Kozlov and Callaghan, 2008). The reasons include lower plant defences and higher plant nutritional value in the presence of increased CO2 and O3 (Kopper and Lindroth, 2003) and altered seasonal synchrony between plants, insect herbivores and their natural enemies (van Asch and Visser, 2007; Stireman et al., 2005).
Many insects are sensitive to extreme weather events (droughts, heat waves, cold spells). As a result of climate change and deforestation, tropical environments that harbour the bulk of Earth’s biodiversity could very well become too hot, dry or fragmented for many insect species to persist (Williams, Bolitho and Fox, 2003). Species that exhibit highly evolved host plant interactions or inhabit microhabitats are at high risk of extinction, especially in tropical areas (Lewis, 2006).
The climates of temperate and subarctic regions are becoming increasingly hospitable to plant and insect life, raising concerns about the behaviour of indigenous species and about the risk of invasion by exotic species, which could result in disruption of normal ecosystem functions. Many temperate-zone insect species have shifted their distributions in response to recent climate change. Examples are the pine processionary moth (Thaumetopoea pityocampa) in Europe (Battisti et al., 2006), winter moth (Operophtera brumata) and autumnal moth (Epirrita autumnata) in Scandinavia (Jepsen et al., 2008) and southern pine beetle (Dendroctonus frontalis) in North America (Tran et al., 2007). Some species that have historically been constrained in their distribution by geographical barriers such as mountain ranges or large bodies of water are likely to overcome these barriers and suddenly expand their range. For example, increased movements of warm air masses towards high latitudes have caused recent influxes of diamondback moth (Plutella xylostella) on the Norwegian islands of Svalbard in the Arctic Ocean, 800 km north of the edge if its current distribution in the western Russian Federation (Coulson et al., 2002).
The fate of specific insect species depends on their degree of specialization (host and habitat range), their mobility and the factors constraining their distribution. Specialist butterfly species are declining in abundance in the United Kingdom, while generalist species are increasing (Thomas, 2005; Franco et al., 2006). Insect species richness is increasing in the cool habitats of the planet (Andrew and Hughes, 2005). Butterfly species found throughout the United Kingdom are decreasing most rapidly in the south, while species with a southerly distribution are expanding northward (Conrad et al., 2004). Thus, the geographical range of insect species may be shifting by simultaneous expansion at the upper end and contraction at the lower end of their latitude and altitude limits (Parmesan et al., 1999).
Insect species are also changing their genetic makeup in response to climate change. While genetic change is a normal process in nature, exceptionally rapid alterations have been observed over short periods (in the order of a decade) in morphology related to flight capacity (Hill, Thomas and Blakeley, 1999; Thomas et al., 2001), life history strategies, diapause (dormancy) induction (Burke et al., 2005), developmental physiology (Rank and Dahlhoff, 2002) and cold tolerance (Calosi et al., 2008) in species that are changing their range.
Conclusive evidence of changes in outbreak frequencies among forest insect pests in response to climate change is rare, as it must be based on long historical records and adequate knowledge of each insect’s population dynamics. Considerable information has linked drought stress due to climate change and extensive damage by insects to pinyon pine (Pinus spp.) in the southwestern United States (Trotter, Cobb and Whitham, 2008). There is evidence that the regular (8- to13-year) outbreak cycles of larch budmoth (Zeiraphera diniana) in Switzerland have stopped since the early 1970s (Esper et al., 2007). Outbreaks of spruce budworm (Choristoneura spp.) in eastern Canada seem to have increased in frequency and severity over the past 200 years (Simard, Morin and Lavoie, 2006). Climate change can affect the behaviour of insect populations in their current range by altering the ecological interactions that regulate them. These effects are difficult to predict in large part because the population dynamics of few species are sufficiently understood (Harrington, Fleming and Woiwod, 2001). Even for the most studied species, such as the spruce budworm in North America, the complexity of ecological interactions involved is almost overwhelming (Eveleigh et al., 2007).
Global spread of harmful forest pest species is a possible consequence of climate change. Because of the diverse and complex responses of insects to climatic factors, it is difficult to make general predictions. Generic modelling tools such as BioSIM (Régnière and St-Amant, 2008) use available knowledge about the responses of particular species (usually pests) to key climatic factors to predict their potential geographic range and performance. These models focus mainly on factors that determine the insect’s seasonality and those that affect its survival during the harshest season (usually winter). They are based on the idea that the insect’s most fundamental requirement is to complete the life cycle in a well-adapted seasonal pattern, with adequate synchrony between essential resources such as host plants for food and shelter and the life stages that require them. If a species cannot satisfy this basic viability requirement generation after generation under a specific climate, it cannot persist in that environment.
Once a seasonality model is available for an insect species, its distribution can be predicted by mapping climates that produce viable seasonality with more or less certainty, and overlaying the distribution of resources vital to (or most at risk from) that species. Predictions can be further refined by also considering the probability of survival under extreme climatic conditions (based for example on tolerance to cold or heat). This approach has been applied to three species of importance to North American forests, using climate normals (averages and variances measured over standard 30-year intervals) for the periods 1971–2000 and 2041–2070 based on a conservative climate change scenario driven by a 1 percent per year increase in atmospheric CO2 (Logan, Régnière and Powell, 2003).
The spruce budworm Choristoneura fumiferana is a native defoliator of conifers that ranges from the West to East Coasts in Canada and through the northern United States from Minnesota in the Midwest to Maine on the East Coast. The northern part of its current distribution is generally limited by the range of its host plants. However, there are areas in eastern Canada where it is limited by adverse climate and where climate change is likely to allow it to thrive. The analysis used a detailed process-based model of the insect’s developmental responses to temperature. Winter cold is not a particularly important source of mortality for spruce budworm; this species, which is strictly univoltine (i.e. has one brood per year), spends winter in a deep diapause in early larval development. Where host plants are available, its northern and high altitude limit is set by summers that are too cool for eggs to hatch before winter, in time for larvae to find appropriate winter shelter. Thus, the probability of egg hatch before the onset of winter is a good estimator of the insect’s likelihood of persisting in a given location. The models depict accurately the insect’s current distribution in eastern Canada. Under climate change, this distribution is expected to creep towards more northerly latitudes and higher altitudes, to be limited only by the availability of suitable host trees (Figure 1). There is evidence that this is already occurring, as a new outbreak is developing at unusually high latitudes on the north shore of the Saint Lawrence River in Quebec. More severe and prolonged outbreaks can therefore be expected in areas that have usually escaped such damage in the past because of inclement weather.
The gypsy moth (Lymantria dispar) was introduced from Europe to the northeastern United States in 1869 and has spread west and south in the United States and north into Canada where it has now reached its northern limit set by adverse climatic conditions. Currently, the gypsy moth is confined to the east of Lake Superior (Figure 2). A model of the insect’s seasonality was used to predict the probability of its establishment in Canada (Régnière, Nealis and Porter, 2009). The model predicted that this species, which is highly polyphagous (i.e. has many host plants), will threaten considerable hardwood forest resources as climate change allows it to expand further north and west into Canada. It is estimated that the proportion of Canada’s deciduous forests at risk of damage by gypsy moth will grow from the current 15 percent to more than 75 percent by 2050. Much of the management strategy to reduce this risk involves monitoring and control west of Lake Superior, which has been a geographical barrier to the insect’s northern spread route between Ontario and Manitoba, in conjunction with the Slow the Spread programme in the midwestern United States to prevent spread from the south.
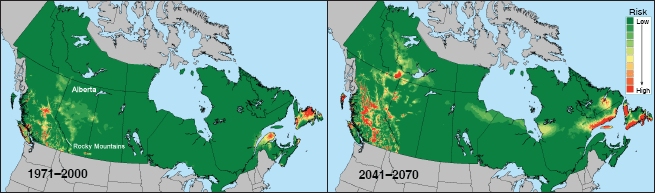
The mountain pine beetle, Dendroctonus ponderosae, is an indigenous North American bark beetle that has been confined to the west of the continent by the Rocky Mountains and the Great Plains geographical barriers. Considerable knowledge of the insect’s physiological responses to temperature is available, both in terms of development (Bentz, Logan and Amman, 1991) and cold tolerance (Régnière and Bentz, 2007). Models were used to determine the area in Canada that is climatically most suitable to this insect. Exactly one generation per year is ideal for this diapause-free species. Risk maps developed for the country overlay the probabilities that the insect can achieve an adaptive seasonality and that it can survive the extreme cold of the Canadian winters, both under current and future climates (Figure 3). These maps suggest that Canada east of the Rocky Mountains will remain inhospitable to this insect well into the future, except for parts of northwestern Alberta and the Atlantic seaboard. However, the risk of mountain pine beetle outbreaks in the western part of the country is likely to increase dramatically towards higher latitudes and altitudes, while decreasing at lower latitudes and altitudes. This information, combined with knowledge of the susceptibility of the various pine (Pinus) species in Canada’s boreal forest, helped Canada devise a risk-based strategy for managing an unprecedented outbreak of mountain pine beetle in British Columbia and Alberta (Nealis and Peter, 2008) [Ed. note: see article by Konkin and Hopkins, this issue].
| 1 Probability of spruce budworm egg hatch prior to the onset of winter in Quebec, Canada under current and predicted future climate |
 |
| 2 Probability of gypsy moth establishment in Canada under current and predicted future climate, based on adequate seasonality |
 |
| 3 Risk of mountain pine beetle having a univoltine (one-brood) life cycle and high winter survival in Canada under recent and predicted future climate |
 |
The literature points to a loss of insect biodiversity in the tropical areas of the planet, as highly specific species face the disappearance of suitable climates and hosts. At middle latitudes, distribution shifts towards higher latitudes and altitudes seem to be prevalent, especially in highly mobile and polyphagous species.
Detailed models of the responses to climate of each insect species are needed to predict distribution changes with any accuracy. However, it seems difficult to make general predictions about the responses of major forest insect pest species from the point of view of outbreak severity and frequency in their current ranges. There is an increasing risk of “invasion” into increasingly hospitable temperate ecosystems by the more mobile species. However, models indicate that insect distributions should not be expected to expand, but rather to shift towards higher latitudes and altitudes. Thus a warmer world does not necessarily imply a more pestilent world.
![]() Bibliography
Bibliography
Andrew, N.R. & Hughes, L. 2005. Diversity and assemblage structure of phytophagous Hemiptera along a latitudinal gradient: predicting the potential impacts of climate change. Global Ecology and Biogeography, 14: 249–262.
Battisti, A., Stastny, M., Buffo, E. & Larsson, S. 2006. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Global Change Biology, 12: 662–671.
Bentz, B.J., Logan, J.A. & Amman, G.D. 1991. Temperature-dependent development of the mountain pine beetle (Coleoptera: Scolytidae) and simulation of its phenology. The Canadian Entomologist, 123: 1083–1094.
Burke, S., Pulin, A.S., Wilson, R.J. & Thomas, C.D. 2005. Selection of discontinuous life-history traits along a continuous thermal gradient in the butterfly Aricia agestis. Ecological Entomology, 30: 613–619.
Calosi, P., Bilton, D.T., Spicer, J.I. & Atfield, A. 2008. Thermal tolerance and geographical range size in the Agabus brunneus group of European diving beetles (Coleptera: Dytiscidae). Journal of Biogeography, 35: 295–305.
Conrad, K.F., Woiwod, I.P., Parsons, M., Fox, R. & Warren, M.S. 2004. Long-term population trends in widespread British moths. Journal of Insect Conservation, 8: 119–136.
Coulson, S.J., Hodkinson, I.D., Webb, N.R., Mikkola, K., Harrison, J.A. & Pedgley, D.E. 2002. Aerial colonization of high Arctic islands by invertebrates: the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) as a potential indicator species. Diversity and Distributions, 8: 327–334.
Currano, E.D., Wilf, P., Wing, S.L., Labandeira, C.C., Lovelock, E.C. & Royer, D.L. 2008. Sharply increased insect herbivory during the paleocene-eocene thermal maximum. Proceedings of the National Academy of Sciences, 105: 1960–1964.
DeLucia, E.H., Casteel, C.L., Nabity, P.D. & O’Neill, B.F. 2008. Insects take a bigger bite out of plants in a warmer, higher carbon dioxide world. Proceedings of the National Academy of Sciences, 105: 1781–1782.
Esper, J., Büntgen, U., Frank, D.C., Nievergelt, D. & Liebhold, A. 2007. 1200 years of regular outbreaks in alpine insects. Proceedings of the Royal Society Series B, 274: 671–679.
Eveleigh, E.S., McCann, K.S., McCarthy, P.C., Pollock, S.J., Lucarotti, C.J., Morin, B., McDougall, G.A., Strongman, D.B., Huber, J.T., Umbanhowar, J. & Faria, L.B.D. 2007. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proceedings of the National Academy of Sciences, 104: 16976–16981.
Franco, A.M.A., Hill, J.K., Kitschke, C., Collingham, Y.C., Roy, D.B., Fox, R., Huntley, B. & Thomas, C.D. 2006. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Global Change Biology, 12(8): 1545–1553.
Harrington, R., Fleming, R.A. & Woiwod, I.P. 2001. Climate change impacts on insect management and conservation in temperate regions: can they be predicted? Agricultural and Forest Entomology, 3: 233–240.
Hill, J.K., Thomas, C.D. & Blakeley, D.S. 1999. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia, 121: 165–170.
Jepsen, J.U., Hagen, S.B., Ims, R.A. & Yoccoz, N.G. 2008. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. Journal of Animal Ecology, 77: 257–264.
Kopper, B.J. & Lindroth, R.L. 2003. Effects of elevated carbon dioxide and ozone on the phytochemistry of aspen and performance of an herbivore. Oecologia, 134: 95–103.
Lewis, S.L. 2006. Tropical forests and the changing earth system. Philosophical transactions of the Royal Society B, 361: 195–210.
Logan, J.A., Régnière, J. & Powell, J.A. 2003. Assessing the impact of global warming on forest pest dynamics. Frontiers of Ecology and the Environment, 1(3): 130–137.
Moore, B. & Allard, G. 2008. Climate change impacts on forest health. Forest Health and Biosecurity Working Paper FBS/34E. Rome, FAO.
Nealis, V. & Peter, B. 2008. Risk assessment of the threat of mountain pine beetle to Canada’s boreal and eastern pine forests. Information Report BC-X-417. Victoria, British Columbia, Canada, Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre.
Parmesan, C., Ryrholm, N., Stefanescu, C., Hill, J.K., Thomas, C.D., Descimon, H., Huntley, B., Kaila, L., Kullberg, J., Tammaru, T., Tennent, W.J., Thomas, J.A. & Warren, M. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature, 399: 579–583.
Rank, N.E. & Dahlhoff, E.P. 2002. Allele frequency shifts in response to climate change and physiological consequences of allozyme variation in a montane insect. Evolution, 56: 2278–2289.
Régnière, J. & Bentz, B. 2007. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. Journal of Insect Physiology, 53: 559–572.
Régnière, J., Nealis, V. & Porter, K. 2009. Climate suitability and management of the gypsy moth invasion into Canada. Biological Invasions, 11: 135–148.
Régnière, J. & St-Amant, R. 2008. BioSIM 9 user’s manual. Information Report LAU-X-134. Quebec, Canada, Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre.
Simard, I., Morin, H. & Lavoie, C. 2006. A millenial-scale reconstruction of spruce budworm abundance in Saguenay, Québec, Canada. The Holocene, 16: 31–37.
Stireman, J.O. III, Dyer, L.A., Janzen, D.H., Singer, M.S., Lill, J.T., Marquis, J.R., Ricklefs, R.E., Gentry, G.L., Hallwachs, W., Coley, P.D., Barone, J.A., Greemey, H.F., Connahs, H., Barbosa, P., Morais, H.C. & Diniz, I.R. 2005. Climatic unpredictability and parasitism of caterpillars: implications of global warming. Proceedings of the National Academy of Sciences, 102: 17384–17386.
Thomas, C.D., Bodsworth, E.J., Wilson, R.J., Simmons, A.D., Davies, Z.G., Musche, M. & Conradt, L. 2001. Ecological and evolutionary processes at expanding range margins. Nature, 411: 577–581.
Thomas, J.A. 2005. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philosophical Transactions of the Royal Society B, 360: 339–357.
Tran, J.K., Ylioja, T., Billings, R.F., Régnière, J. & Ayres, M.P. 2007. Impact of minimum winter temperatures on the population dynamics of Dendroctonus frontalis. Ecological Applications, 17: 882–899.
Trotter, R.T. III, Cobb, N.S. & Whitham, T.G. 2008. Arthropod community diversity and trophic structure: a comparison between extremes of plants stress. Ecological Entomology, 33: 1–11.
van Asch, M. & Visser, M.E. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annual Review of Entomology, 52: 37–55.
Williams, S.E., Bolitho, E.E. & Fox, S. 2003. Climate change in Australian tropical rainforests: an impending environmental catastrophe. Proceedings of the Royal Society of London B, 270: 1887–1892.
Wolf, A., Kozlov, M.V. & Callaghan, T.V. 2008. Impact of non-outbreak insect damage on vegetation in northern Europe will be greater than expected during a changing climate. Climatic Change, 87: 91–106.