2.3.1. Physical and chemical conditions
2.3.2. Growth dynamics
2.3.3. Isolating/obtaining and maintaining of cultures
2.3.4. Sources of contamination and water treatment
2.3.5. Algal culture techniques
2.3.6. Algal production in outdoor ponds
2.3.7. Culture of sessile micro-algae
2.3.8. Quantifying algal biomass
2.3.9. Harvesting and preserving micro-algae
2.3.10. Algal production cost
2.3.1.1. Culture medium/nutrients
2.3.1.2. Light
2.3.1.3. pH
2.3.1.4. Aeration/mixing
2.3.1.5. Temperature
2.3.1.6. Salinity
The most important parameters regulating algal growth are nutrient quantity and quality, light, pH, turbulence, salinity and temperature. The most optimal parameters as well as the tolerated ranges are species specific and a broad generalization for the most important parameters is given in Table 2.2. Also, the various factors may be interdependent and a parameter that is optimal for one set of conditions is not necessarily optimal for another.
Concentrations of cells in phytoplankton cultures are generally higher than those found in nature. Algal cultures must therefore be enriched with nutrients to make up for the deficiencies in the seawater. Macronutrients include nitrate, phosphate (in an approximate ratio of 6:1), and silicate.
Table 2.2. A generalized set of conditions for culturing micro-algae (modified from Anonymous, 1991).
|
Parameters |
Range |
Optima |
|
Temperature (°C) |
16-27 |
18-24 |
|
Salinity (g.l-1) |
12-40 |
20-24 |
|
Light intensity (lux) |
1,000-10,000 |
2,500-5,000 |
|
Photoperiod (light: dark, hours) |
|
16:8 (minimum) |
|
pH |
7-9 |
8.2-8.7 |
Table 2.3. Composition and preparation of Walne medium (modified from Laing, 1991).
|
Constituents |
Quantities |
|
Solution A (at 1 ml per liter of culture) |
|
|
Ferric chloride (FeCl3) |
0.8 g(a) |
|
Manganous chloride (MnCl2,
4H2O) |
0.4 g |
|
Boric acid (H3BO3) |
33.6 g |
|
EDTA(b), di-sodium salt |
45.0 g |
|
Sodium di-hydrogen orthophosphate
(NaH2PO4, 2H2O) |
20.0 g |
|
Sodium nitrate (NaNO3) |
100.0 g |
|
Solution B |
1.0 ml |
|
Make up to 1 litre with fresh water(c) |
Heat to dissolve |
|
Solution B |
|
|
Zinc chloride (ZnCl2) |
2.1 g |
|
Cobaltous chloride (CoCl2,6
H2O) |
2.0 g |
|
Ammonium molybdate
((NH4)6Mo7O24,
4H2O) |
0.9 g |
|
Cupric sulphate (CuSO4, 5H2O) |
2.0 g |
|
Concentrated HCl |
10.0 ml |
|
Make up to 100 ml fresh water(c) |
Heat to dissolve |
|
Solution C (at 0.1 ml per liter of culture) |
|
|
Vitamin B1 |
0.2 g |
|
Solution E |
25.0 ml |
|
Make up to 200 ml with fresh water(c) |
|
|
Solution D (for culture of diatoms-used in addition to
solutions A and C, at 2 ml per liter of culture) |
|
|
Sodium metasilicate (Na2SiO3,
5H2O) |
40.0 g |
|
Make up to 1 litre with fresh water(c) |
Shake to dissolve |
|
Solution E |
|
|
Vitamin B12 |
0.1 g |
|
Make up to 250 ml with fresh water(c) |
|
|
Solution F (for culture of Chroomonas salina - used
in addition to solutions A and C, at 1 ml per liter of culture) |
|
|
Sodium nitrate (NaNO3) |
200.0 g |
|
Make up to 1 litre with fresh water(c) |
|
(a) Use 2.0 g for culture of Chaetoceros calcitrans in filtered sea water;
(b) Ethylene diamine tetra acetic acid;
(c) Use distilled water if possible.
Table 2.4. Composition and preparation of Guillard’s F/2 medium (modified from Smith et al., 1993a).
|
Nutrients |
Final concentration |
Stock solution preparations |
|
NaNO3 |
75 |
Nitrate/Phosphate Solution |
|
NaH2PO4.H2O |
5 |
|
|
Na2SiO3.9H2O |
30 |
Silicate Solution |
|
Na2C10H14O8N2.H2O
(Na2EDTA) |
4.36 |
Trace Metal/EDTA Solution |
|
CoCl2.6H2O |
0.01 |
1-liter stocks of (g.l-1 DW) 10.0 g
CoCl2, 9.8 g |
|
CuSO4.5H2O |
0.01 |
CuSO4, 180 g MnCl2, 6.3 g
Na2MoO4, 22.0 g ZnSO4 |
|
FeCl3.6H2O |
3.15 |
|
|
MnCl2.4H2O |
0.18 |
Working stock: add 1 ml of each primary stock solution + 4.35
g Na2C10H14O8N2 + 3.15 g
FeCl3 to 1 liter DW |
|
Na2MoO4.2H2O |
0.006 |
|
|
ZnSO4.7H2O |
0.022 |
|
|
Thiamin HCl |
0.1 |
Vitamin Solution |
|
Biotin |
0.0005 |
|
|
B12 |
0.0005 |
Working stock: add 5 ml primary stock to 1 liter DW |
|
Fertilizers |
Concentration (mg.l-1) |
|||||
|
A |
B |
C |
D |
E |
F |
|
|
Ammonium sulfate |
150 |
100 |
300 |
100 |
- |
- |
|
Urea |
7.5 |
5 |
- |
10-15 |
- |
12-15 |
|
Calcium superphosphate |
25 |
15 |
50 |
- |
- |
- |
|
Clewat 32 |
- |
5 |
- |
- |
- |
- |
|
N:P 16/20 fertilizer |
- |
- |
- |
10-15 |
- |
- |
|
N:P:K 16-20-20 |
- |
- |
- |
- |
12-15 |
- |
|
N:P:K 14-14-14 |
- |
- |
- |
- |
- |
30 |
As with all plants, micro-algae photosynthesize, i.e. they assimilate inorganic carbon for conversion into organic matter. Light is the source of energy which drives this reaction and in this regard intensity, spectral quality and photoperiod need to be considered. Light intensity plays an important role, but the requirements vary greatly with the culture depth and the density of the algal culture: at higher depths and cell concentrations the light intensity must be increased to penetrate through the culture (e.g. 1,000 lux is suitable for erlenmeyer flasks, 5,000-10,000 is required for larger volumes). Light may be natural or supplied by fluorescent tubes. Too high light intensity (e.g. direct sun light, small container close to artificial light) may result in photo-inhibition. Also, overheating due to both natural and artificial illumination should be avoided. Fluorescent tubes emitting either in the blue or the red light spectrum should be preferred as these are the most active portions of the light spectrum for photosynthesis. The duration of artificial illumination should be minimum 18 h of light per day, although cultivated phytoplankton develop normally under constant illumination.
The pH range for most cultured algal species is between 7 and 9, with the optimum range being 8.2-8.7. Complete culture collapse due to the disruption of many cellular processes can result from a failure to maintain an acceptable pH. The latter is accomplished by aerating the culture (see below). In the case of high-density algal culture, the addition of carbon dioxide allows to correct for increased pH, which may reach limiting values of up to pH 9 during algal growth.
Mixing is necessary to prevent sedimentation of the algae, to ensure that all cells of the population are equally exposed to the light and nutrients, to avoid thermal stratification (e.g. in outdoor cultures) and to improve gas exchange between the culture medium and the air. The latter is of primary importance as the air contains the carbon source for photosynthesis in the form of carbon dioxide. For very dense cultures, the CO2 originating from the air (containing 0.03% CO2) bubbled through the culture is limiting the algal growth and pure carbon dioxide may be supplemented to the air supply (e.g. at a rate of 1% of the volume of air). CO2 addition furthermore buffers the water against pH changes as a result of the CO2/HCO3- balance. Depending on the scale of the culture system, mixing is achieved by stirring daily by hand (test tubes, erlenmeyers), aerating (bags, tanks), or using paddle wheels and jetpumps (ponds). However, it should be noted that not all algal species can tolerate vigorous mixing.
The optimal temperature for phytoplankton cultures is generally between 20 and 24°C, although this may vary with the composition of the culture medium, the species and strain cultured. Most commonly cultured species of micro-algae tolerate temperatures between 16 and 27°C. Temperatures lower than 16°C will slow down growth, whereas those higher than 35°C are lethal for a number of species. If necessary, algal cultures can be cooled by a flow of cold water over the surface of the culture vessel or by controlling the air temperature with refrigerated air - conditioning units.
Marine phytoplankton are extremely tolerant to changes in salinity. Most species grow best at a salinity that is slightly lower than that of their native habitat, which is obtained by diluting sea water with tap water. Salinities of 20-24 g.l-1 have been found to be optimal.
The growth of an axenic culture of micro-algae is characterized by five phases (Fig. 2.3.):
· lag or induction phase
This phase, during which little increase in cell density occurs, is relatively long when an algal culture is transferred from a plate to liquid culture. Cultures inoculated with exponentially growing algae have short lag phases, which can seriously reduce the time required for upscaling. The lag in growth is attributed to the physiological adaptation of the cell metabolism to growth, such as the increase of the levels of enzymes and metabolites involved in cell division and carbon fixation.
Figure 2.3. Five growth phases of micro-algae cultures.
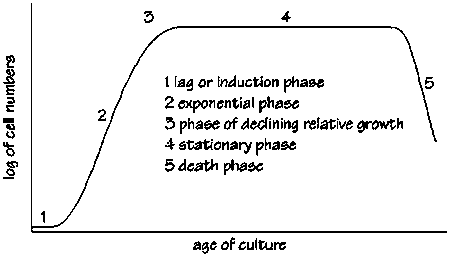
· exponential phase
During the second phase, the cell density increases as a function of time t according to a logarithmic function:
Ct = C0.emt
with Ct and C0 being the cell concentrations at time t and 0, respectively, and m = specific growth rate. The specific growth rate is mainly dependent on algal species, light intensity and temperature.
· phase of declining growth rate
Cell division slows down when nutrients, light, pH, carbon dioxide or other physical and chemical factors begin to limit growth.
· stationary phase
In the fourth stage the limiting factor and the growth rate are balanced, which results in a relatively constant cell density.
· death or “crash” phase
During the final stage, water quality deteriorates and nutrients are depleted to a level incapable of sustaining growth. Cell density decreases rapidly and the culture eventually collapses.
In practice, culture crashes can be caused by a variety of reasons, including the depletion of a nutrient, oxygen deficiency, overheating, pH disturbance, or contamination. The key to the success of algal production is maintaining all cultures in the exponential phase of growth. Moreoever, the nutritional value of the produced algae is inferior once the culture is beyond phase 3 due to reduced digestibility, deficient composition, and possible production of toxic metabolites.
Sterile cultures of micro-algae used for aquaculture purposes may be obtained from specialized culture collections. A list of culture collections is provided by Vonshak (1986) and Smith et al. (1993a). Alternatively, the isolation of endemic strains could be considered because of their ability to grow under the local environmental conditions. Isolation of algal species is not simple because of the small cell size and the association with other epiphytic species. Several laboratory techniques are available for isolating individual cells, such as serial dilution culture, successive plating on agar media (See Worksheet 2.1), and separation using capillary pipettes. Bacteria can be eliminated from the phytoplankton culture by washing or plating in the presence of antibiotics. The sterility of the culture can be checked with a test tube containing sea water with 1 g.l-1 bactopeptone. After sterilization, a drop of the culture to be tested is added and any residual bacteria will turn the bactopeptone solution turbid.
The collection of algal strains should be carefully protected against contamination during handling and poor temperature regulation. To reduce risks, two series of stocks are often retained, one which supplies the starter cultures for the production system and the other which is only subjected to the handling necessary for maintenance. Stock cultures are kept in test tubes at a light intensity of about 1000 lux and a temperature of 16 to 19°C. Constant illumination is suitable for the maintenance of flagellates, but may result in decreased cell size in diatom stock cultures. Stock cultures are maintained for about a month and then transferred to create a new culture line (Fig. 2.4.).
Contamination with bacteria, protozoa or another species of algae is a serious problem for monospecific/axenic cultures of micro-algae. The most common sources of contamination include the culture medium (sea water and nutrients), the air (from the air supply as well as the environment), the culture vessel, and the starter culture.
Seawater used for algal culture should be free of organisms that may compete with the unicellular algae, such as other species of phytoplankton, phytophagous zooplankton, or bacteria. Sterilization of the seawater by either physical (filtration, autoclaving, pasteurization, UV irradiation) or chemical methods (chlorination, acidification, ozonization) is therefore required. Autoclaving (15 to 45 min. at 120°C and 20 psi, depending on the volume) or pasteurization (80°C for 1-2 h) is mostly applied for sterilizing the culture medium in test tubes, erlenmeyers, and carboys. Volumes greater than 20 l are generally filtered at 1 µm and treated with acid (e.g. hydrochloric acid at pH 3, neutralization after 24 h with sodium carbonate) or chlorine (e.g. 1-2 mg.l-1, incubation for 24 h without aeration, followed by aeration for 2-3 h to remove residual chlorine, addition of sodium thiosulfate to neutralize chlorine may be necessary if aeration fails to strip the chlorine). Water treatment is not required when using underground salt water obtained through bore holes. This water is generally free of living organisms and may contain sufficient mineral salts to support algal culture without further enrichment. In some cases well water contains high levels of ammonia and ferrous salts, the latter precipitating after oxidation in air.
A common source of contamination is the condensation in the airlines which harbor ciliates. For this reason, airlines should be kept dry and both the air and the carbon dioxide should be filtered through an in-line filter of 0.3 or 0.5 µm before entering the culture. For larger volumes of air, filter units can be constructed using cotton and activated charcoal (Fig.2.5.).
Figure 2.5. Aeration filter (Fox, 1983)

The preparation of the small culture vessels is a vital step in the upscaling of the algal cultures:
· wash with detergent
· rinse in hot water
· clean with 30% muriatic acid
· rinse again with hot water
· dry before use.
Alternatively, tubes, flasks and carboys can be sterilized by autoclaving and disposable culture vessels such as polyethylene bags can be used.
2.3.5.1. Batch culture
2.3.5.2. Continuous culture
2.3.5.3. Semi-continuous culture
Algae can be produced using a wide variety of methods, ranging from closely-controlled laboratory methods to less predictable methods in outdoor tanks. The terminology used to describe the type of algal culture include:
· Indoor/Outdoor. Indoor culture allows control over illumination, temperature, nutrient level, contamination with predators and competing algae, whereas outdoor algal systems make it very difficult to grow specific algal cultures for extended periods.· Open/Closed. Open cultures such as uncovered ponds and tanks (indoors or outdoors) are more readily contaminated than closed culture vessels such as tubes, flasks, carboys, bags, etc.
· Axenic (=sterile)/Xenic. Axenic cultures are free of any foreign organisms such as bacteria and require a strict sterilization of all glassware, culture media and vessels to avoid contamination. The latter makes it impractical for commercial operations.
· Batch, Continuous, and Semi-Continuous. These are the three basic types of phytoplankton culture which will be described in the following sections.
Table 2.6. summarizes the major advantages and disadvantages of the various algal culture techniques.
Table 2.6. Advantages and disadvantages of various algal culture techniques (modified from Anonymous, 1991).
|
Culture type |
Advantages |
Disadvantages |
|
Indoors |
A high degree of control (predictable) |
Expensive |
|
Outdoors |
Cheaper |
Little control (less predictable) |
|
Closed |
Contamination less likely |
Expensive |
|
Open |
Cheaper |
Contamination more likely |
|
Axenic |
Predictable, less prone to crashes |
Expensive, difficult |
|
Non-axenic |
Cheaper, less difficult |
More prone to crashes |
|
Continuous |
Efficient, provides a consistent supply of high-quality cells,
automation, highest rate of production over extended periods |
Difficult, usually only possible to culture small quantities,
complex, equipment expenses may be high |
|
Semi-continuous |
Easier, somewhat efficient |
Sporadic quality, less reliable |
|
Batch |
Easiest, most reliable |
Least efficient, quality may be inconsistent |
The batch culture consists of a single inoculation of cells into a container of fertilized seawater followed by a growing period of several days and finally harvesting when the algal population reaches its maximum or near-maximum density. In practice, algae are transferred to larger culture volumes prior to reaching the stationary phase and the larger culture volumes are then brought to a maximum density and harvested. The following consecutive stages might be utilized: test tubes, 2 l flasks, 5 and 20 l carboys, 160 l cylinders, 500 l indoor tanks, 5,000 l to 25,000 l outdoor tanks (Figs. 2.6., 2.7).
Table 2.7. Inoculation schedule for the continuous production of micro-algae using the batch technique. Every week a serial is initiated with 4 or 7 test tubes, depending on whether a new culture is required for harvesting every 2 days or daily.
|
Days |
New culture available for harvest every 2
days |
Harvest required daily |
|||||||||
|
1 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
2 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
3 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
4 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
5 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
6 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
7 |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
t |
|
8 |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
|
9 |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
|
10 |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
|
11 |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
e |
|
12 |
E |
e |
e |
e |
E |
e |
e |
e |
e |
e |
e |
|
13 |
E |
e |
e |
e |
E |
E |
e |
e |
e |
e |
e |
|
14 |
E |
E |
e |
e |
E |
E |
E |
e |
e |
e |
e |
|
15 |
E |
E |
e |
e |
E |
E |
E |
E |
e |
e |
e |
|
16 |
f |
E |
E |
e |
f |
E |
E |
E |
E |
e |
e |
|
17 |
f |
E |
E |
e |
f |
f |
E |
E |
E |
E |
e |
|
18 |
f |
f |
E |
E |
f |
f |
f |
E |
E |
E |
E |
|
19 |
f |
f |
E |
E |
f |
f |
f |
f |
E |
E |
E |
|
20 |
F |
f |
f |
E |
F |
f |
f |
f |
f |
E |
E |
|
21 |
F |
f |
f |
E |
F |
F |
f |
f |
f |
f |
E |
|
22 |
F |
F |
f |
f |
F |
F |
F |
f |
f |
f |
f |
|
23 |
F |
F |
f |
f |
F |
F |
F |
F |
f |
f |
f |
|
24 |
L |
F |
F |
f |
L |
F |
F |
F |
F |
f |
f |
|
25 |
L |
F |
F |
f |
L |
L |
F |
F |
F |
F |
f |
|
26 |
* |
L |
F |
F |
* |
L |
L |
F |
F |
F |
F |
|
27 |
|
L |
F |
F |
|
* |
L |
L |
F |
F |
F |
|
28 |
|
* |
L |
F |
|
|
* |
L |
L |
F |
F |
|
29 |
|
|
L |
F |
|
|
|
* |
L |
L |
F |
|
30 |
|
|
* |
L |
|
|
|
|
* |
L |
L |
|
31 |
|
|
|
L |
|
|
|
|
|
* |
L |
|
32 |
|
|
|
* |
|
|
|
|
|
|
* |
t = 20 ml test tube
e = 250 ml erlenmeyer flask
E = 2 l erlenmeyer flask
f = 30 l fiberglass tank
F = 300 l fiberglass tank
L = use for larval feeding or to inoculate large volume (> 1.5 t) outdoor tanks
* = termination of 300 l fiberglass tank
Figure 2.6. Production scheme for batch culture of algae (Lee and Tamaru, 1993).

According to the algal concentration, the volume of the inoculum which generally corresponds with the volume of the preceding stage in the upscaling process, amounts to 2-10% of the final culture volume. An inoculation schedule for the continuous production according to the batch technique is presented in Table 2.7. Where small amounts of algae are required, one of the simplest types of indoor culture employs 10 to 20 l glass or plastic carboys (Fig. 2.8.), which may be kept on shelves backlit with fluorescent tubes (Fig. 2.9.).
Batch culture systems are widely applied because of their simplicity and flexibility, allowing to change species and to remedy defects in the system rapidly. Although often considered as the most reliable method, batch culture is not necessarily the most efficient method. Batch cultures are harvested just prior to the initiation of the stationary phase and must thus always be maintained for a substantial period of time past the maximum specific growth rate. Also, the quality of the harvested cells may be less predictable than that in continuous systems and for example vary with the timing of the harvest (time of the day, exact growth phase).
Another disadvantage is the need to prevent contamination during the initial inoculation and early growth period. Because the density of the desired phytoplankton is low and the concentration of nutrients is high, any contaminant with a faster growth rate is capable of outgrowing the culture. Batch cultures also require a lot of labour to harvest, clean, sterilize, refill, and inoculate the containers.
Figure 2.7.a. Batch culture systems for the mass production of micro-algae in 20,000 l tanks.
Figure 2.7.b. Batch culture systems for the mass production of micro-algae in 150 l cylinders.
Figure 2.8. Carboy culture apparatus (Fox, 1983).

Figure 2.9. Carboy culture shelf (Fox, 1983).
The continuous culture method, (i.e. a culture in which a supply of fertilized seawater is continuously pumped into a growth chamber and the excess culture is simultaneously washed out), permits the maintenance of cultures very close to the maximum growth rate. Two categories of continuous cultures can be distinguished:
· turbidostat culture, in which the algal concentration is kept at a preset level by diluting the culture with fresh medium by means of an automatic system.· chemostat culture, in which a flow of fresh medium is introduced into the culture at a steady, predetermined rate. The latter adds a limiting vital nutrient (e.g. nitrate) at a fixed rate and in this way the growth rate and not the cell density is kept constant.
Laing (1991) described the construction and operation of a 40 l continuous system suitable for the culture of flagellates, e.g. Tetraselmis suecica and Isochrysis galbana (Fig. 2.10.). The culture vessels consist of internally-illuminated polyethylene tubing supported by a metal framework (Fig. 2.11.). This turbidostat system produces 30-40 l per day at cell densities giving optimal yield for each flagellate species (Table 2.8.). A chemostat system that is relatively easy and cheap to construct is utilized by Seasalter Shellfish Co. Ltd, UK (Fig. 2.12.). The latter employ vertical 400 l capacity polyethylene bags supported by a frame to grow Pavlova lutheri, Isochrysis galbana, Tetraselmis suecica, Phaeodactylum tricornutum, Dunaliella tertiolecta, Skeletonema costatum. One drawback of the system is the large diameter of the bags (60 cm) which results in self-shading and hence relatively low algal densities.
The disadvantages of the continuous system are its relatively high cost and complexity. The requirements for constant illumination and temperature mostly restrict continuous systems to indoors and this is only feasible for relatively small production scales. However, continuous cultures have the advantage of producing algae of more predictable quality. Furthermore, they are amenable to technological control and automation, which in turn increases the reliability of the system and reduces the need for labor.
Figure 2.10. Diagram of a continuous culture apparatus (not drawn to scale): (1) enriched seawater medium reservoir (200 l); (2) peristaltic pump; (3) resistance sensing relay (50 - 5000 ohm); (4) light-dependent resistor (ORP 12); (5) cartridge filter (0.45 mm); (6) culture vessel (40 l); (7) six 80 W fluorescent tubes (Laing, 1991).

Figure 2.11. Schematic diagram of a 40 l continuous culture vessel (Laing, 1991).


Table 2.8. Continuous culture methods for various types of algae in 40 l internally-illuminated vessels (suitable for flagellates only) (modified from Laing, 1991),
|
Algae |
Culture density for highest yield |
Usual life of culture |
|
Tetraselmis suecica |
2 000 |
3-6 |
|
Chroomonas salina |
3 000 |
2-3 |
|
Dunaliella tertiolecta |
4 000 |
3-4 |
|
Isochrysis galbana |
|
|
|
Monochrysis lutheri |
|
|
|
Pseudoisochrysis paradoxa |
20 000 |
2-3 |
The semi-continuous technique prolongs the use of large tank cultures by partial periodic harvesting followed immediately by topping up to the original volume and supplementing with nutrients to achieve the original level of enrichment. The culture is grown up again, partially harvested, etc. Semi-continuous cultures may be indoors or outdoors, but usually their duration is unpredictable. Competitors, predators and/or contaminants and metabolites eventually build up, rendering the culture unsuitable for further use. Since the culture is not harvested completely, the semi-continuous method yields more algae than the batch method for a given tank size.
Large outdoor ponds either with a natural bottom or lined with cement, polyethylene or PVC sheets have been used successfully for algal production. The nutrient medium for outdoor cultures is based on that used indoors, but agricultural-grade fertilizers are used instead of laboratory-grade reagents (Table 2.5). However, fertilization of mass algal cultures in estuarine ponds and closed lagoons used for bivalve nurseries was not found to be desirable since fertilizers were expensive and it induced fluctuating algal blooms, consisting of production peaks followed by total algal crashes. By contrast, natural blooms are maintained at a reasonable cell density throughout the year and the ponds are flushed with oceanic water whenever necessary. Culture depths are typically 0.25-1 m. Cultures from indoor production may serve as inoculum for monospecific cultures. Alternatively, a phytoplankton bloom may be induced in seawater from which all zooplankton has been removed by sand filtration. Algal production in outdoor ponds is relatively inexpensive, but is only suitable for a few, fast-growing species due to problems with contamination by predators, parasites and “weed” species of algae. Furthermore, outdoor production is often characterized by a poor batch to batch consistency and unpredictable culture crashes caused by changes in weather, sunlight or water quality.
Mass algal cultures in outdoor ponds are commonly applied in Taiwanese shrimp hatcheries where Skeletonema costatum is produced successfully in rectangular outdoor concrete ponds of 10-40 tons of water volume and a water depth of 1.5-2 m.
Farmers of abalone (Haliotis sp.) have developed special techniques to provide food for the juvenile stages which feed in nature by scraping coralline algae and slime off the surface of rocks using their radulae. In culture operations, sessile micro-algae are grown on plates of corrugated roofing plastic, which serve as a substrate for the settlement of abalone larvae. After metamorphosis, the spat graze on the micro-algae until they become large enough to feed on macro-algae. The most common species of micro-algae used on the feeder plates are pennate diatoms (e.g. Nitzchia, Navicula). The plates are inoculated by placing them in a current of sand filtered seawater. Depending on local conditions, the micro-algae cultures on the plates take between one and three weeks to grow to a density suitable for settling of the larvae. As the spat grow, their consumption rate increases and becomes greater than the natural production of the micro-algae. At this stage, the animals are too fragile to be transferred to another plate and algal growth may be enhanced by increasing illumination intensity and/or by the addition of fertilizer.
There are several ways to evaluate the quantity of algal biomass present in cultures either by counting the number of cells or through determination of volume, optical density or weight.
Cells can be counted either with an electronic particle counter or directly under a microscope, using a haematocytometer. The Coulter® counter and similar instruments need appropriate calibration for each algal species to be counted. Detailed instructions on operation of electronic cell counting can be found in Sheldon and Parsons (1967). The presence of contaminating particles in the same size range as the algae and failure of cells to separate after cell division may be possible sources of erroneous counts. Counting with a microscope has the advantage of allowing control of the quality of the cultures. The major difficulty in microscopic counts is reproducibility, which is a function of the sampling, diluting, and filling of the counting chamber, as well as the choice of the right type of counting chamber and range of cell concentration. Counting chambers, recommended for various cell sizes and concentrations, are listed in Table 2.9. Worksheet 2.2. details on the operation of two types of counting chambers, namely Fuchs-Rosenthal and Bürker.
A relationship between optical density and cellular concentration can be established using a spectrometer. However, variations may occur due to the fact that the chlorophyll concentration in the algal cell varies according to the culture conditions and therefore affects this relationship. In this way, a culture under low lighting conditions will be comparatively more pigmented and will eventually result in higher readings for optical density.
Table 2.9. Cell counting chambers and their properties (modified from Vonshak, 1986).
|
Commercial name of chamber |
Chamber vol |
Depth |
Objective used for magnification |
Cell size |
Cell conc counted easily |
|
Redgwick Rafter |
1.0 |
1.0 |
2.5-10 |
50-100 |
30-104 |
|
Palmer Malony |
0.1 |
0.4 |
10-15 |
5-150 |
102-105 |
|
Speirs Levy |
4.103 |
0.2 |
10-20 |
5-75 |
104-107 |
|
Improved Neaubouer |
2.104 |
0.1 |
20-40 (phase) |
2-30 |
104-107 |
|
Petroff Houser |
2.105 |
0.02 |
40-100 |
0.5-5 |
104-108 |
Measuring the dry weight of a culture is one of the most direct ways to estimate biomass production. For this, the cells of a representative sample of the culture are separated from the culture medium by either centrifugation or filtration on a glassfiber filter. The cells of marine algae are washed with isotonic ammonium formate (0.5 M) to remove salts without causing the cells to burst. Ammonium formate does not leave any residues as it decomposes to volatile compounds during the drying process (e.g. 2 h at 100°C). The results can be expressed as dry weight per volume or, when combined with a determination of the cell concentration, per algal cell (see Worksheet 2.3.).
For a particular algal species, dry weight per cell may vary greatly according to the strain and culture conditions. Published data on the dry weight content for species commonly used in mariculture are presented in Table 2.10. The density of harvested algal cultures generally ranges between 80 and 250 mg of dry weight per liter.
Table 2.10. Cellular dry weight reported in literature for algal species commonly used in mariculture.
|
Algal species |
Dry weight (pg cell -1) |
|
Isochrysis galbana |
8.0, 16.1, 20.1, 23.5, 30.5 |
|
Isochrysis sp. (clone, T-ISO) |
14.1, 17.3, 29.7 |
|
Skeletonema costatum |
52.2 |
|
Thalassiosira pseudonana |
13.2, 17.8, 28.4 |
|
Chaetoceros neogracile (C. gracilis) |
23.8, 30.6, 74.8 |
|
Tetraselmis suecica |
66, 168, 194-244, 247, 292 |
In most cases, it is unnecessary to separate micro-algae from the culture fluid. Excess and off-season production may, however, be concentrated and preserved. The various techniques employed to harvest micro-algae have been reviewed by Fox (1983) and Barnabé (1990). High-density algal cultures can be concentrated by either chemical flocculation or centrifugation. Products such as aluminum sulphate and ferric chloride cause cells to coagulate and precipitate to the bottom or float to the surface. Recovery of the algal biomass is then accomplished by, respectively, siphoning off the supernatant or skimming cells off the surface. Due to the increased particle size, coagulated algae are no longer suitable as food for filter-feeders. Centrifugation of large volumes of algal culture is usually performed using a cream separator; the flow rate being adjusted according to the algal species and the centrifugation rate of the separator. Cells are deposited on the walls of the centrifuge head as a thick algal paste, which is then resuspended in a limited volume of water. The resulting slurry may be stored for 1-2 weeks in the refrigerator or frozen. In the latter case, cryoprotective agents (glucose, dimethylsulfoxide) are added to maintain cell integrity during freezing. However, cell disruption and limited shelf-life remain the major disadvantages of long-term preserved algal biomass. Concentrated cultures of Tetraselmis suecica kept in darkness at 4°C maintain their viability, whereas the latter is completely lost upon freezing. Furthermore, cultures stored in hermetically sealed vials lose their viability more rapidly than those kept in cotton-plugged vials.
Estimates of the algal production cost range from US$ 4 to 300 per kg dry biomass (Table 2.11.). Algal production in outdoor ponds is relatively cheap, but is only suitable for a few, fast-growing species and is characterized by a poor batch-to-batch consistency and unpredictable culture crashes due to contaminations and/or fluctuating climatological conditions. Indoor algal production offers a better control of the culture conditions and the algal species being grown, but is more expensive than outdoor culture due to space, energy, and skilled labour requirements.
An international survey among the operators of bivalve hatcheries showed that only facilities capable of producing mass quantities of specific micro-algae are able to attain production costs below US$ 100 per kg of dry weight (Fig. 2.13.).
Table 2.11. Production cost of marine micro-algae (modified from Coutteau and Sorgeloos, 1992).
|
Production cost |
Remarks |
Source |
|
300 |
Tetraselmis suecica |
calculated from Helm et al. (1979) |
|
167 |
various diatoms |
calculated from Walsh et al. (1987) |
|
4-20 |
outdoor culture |
De Pauw and Persoone (1988) |
|
160-200 |
indoor culture |
|
|
23-115 |
summer-winter production continuous flow cultures in bags (8
m3) and tanks (150 m3)a |
Dravers (pers. comm. 1990) |
|
50 |
tank culture (450 m3)a |
Donaldson (1991) |
|
50 - 400 |
international survey among bivalve hatchery operators in
1991 |
Coutteau and Sorgeloos (1992) |
a total volume available for algal production
Figure 2.13. Algal production cost as a function of the production capacity for 8 bivalve hatcheries. Filled and unfilled symbols represent data obtained from academic and commercial hatcheries, respectively. The dotted line connects the estimates from one company (modified from Coutteau and Sorgeloos, 1992).
