A) PARASITIC PROTOZOA
(J. Lom, I. Dyková)
1. Introduction into the study of protozoan parasites
Discovered already by Leeuwenhoek in late 17th century, protozoa count today about 50 000 species, living in water and soil habitats and also as parasites of animals. Many of them are serious pathogens and several are known as true pests in freshwater or marine aquaculture and in breeding of ornamental fishes.
Protozoa represent a vast assemblage of essentially unicellular organisms, in which a single cell is capable of all life functions executed in higher organisms by special organs. In the system of living organisms they belong to the kingdom Protista. Protozoa grouped into several phyla, Flagellates (phylum Mastigophora), Opalines (Opalinata), Amoebae (Rhizopoda), Coccidia and Piroplasmia (Apicomplexa), Microsporidia (Microspora), Myxosporidia (Myxozoa) and Ciliates (Ciliophora) include agents of serious diseases of fishes.
This chapter will supply a short overwiev on protozoans adapted to the life inside or on the surface of the body of fishes. Emphasis is laid on presentation of most important representatives of various groups of protozoa infecting fishes and on their differentiation. More detailed information on taxonomy, biology and relations to the host can be found in special monographs (see references).
Examining fish for protozoan parasites includes inspection of blood; external surface (skin, fins, nasal pits and gills); internal organs (liver, spleen, kidney, gonads, brain, heart, swimbladder and its rete mirabile, gall and urinary bladders, digestive tract and their content) and musculature.
For an exact, reliable determination of the protozoans, in addition to fresh mounts, and histological sections, special techniques must be applied. They comprise selected staining and silver impregnation methods (Giemsa, Klein), nuclear staining (Feulgen), photomicrography of fresh and stained material and special techniques for characterizing of myxosporean spores.
In order to reveal the true prevalence and intensities of infections fish must be examined as soon as possible after capture. Careful handling during collection and transport minimizes the loss of ectoparasites. To obtain first overall picture of the protozoan fauna the fish sample should comprise at least 10 specimens.
2. Practical key for the determination of fish protozoa in fresh material
| A. | Protozoa can be detected as macroscopical whitish aggregations from tiny dots to cyst-like structures of several mm or even cm in size. On the skin, or gills, or on the internal organs or in their tissue. Determination is only possible by use of a compound microscope. |
| A.1. | Protozoa visible as tiny dots on the body surface and gills. Under the microscope the dot proves to be one or several large (up to 1 mm) slowly rotating cells, uniformly covered with synchronously beating cilia. Next to large ones, there may be small ones of different size. Their cytoplasm is full of granules and contains a large central macronucleus .............................. Ciliates of the genera Ichthyophthirius (freshwater) of Cryptocarion (marine) |
| A.2. | The dot-, nodule-, or cyst-like structures are composed of a mass of small, uniform, refractile bodies (spores or oocysts). |
| A.2.a | The spores, typically 7–20 μm in size, most commonly have 2 (some genera possess 1, 4, 5, 6 or 7) refractile bodies (polar capsules) at their anterior pole .............................. Myxosporea |
| A.2.b | The spores, typically 3–10 μm in size, are usually ellipsoidal or oviform and have a faintly visible vacuole at their posterior end .............................. Microsporidia |
| A.2.c | The spores, typically 3–7 μm in size, are spherical and contain a large centrally located globular refractile inclusion .............................. Dermocystidium |
| A.2.d | The protozoa are spherical or ellipsoidal bodies of about 10–20 μm size, each containing four ellipsoidal bodies each of which contains 2 slender cells. Whitish nodules within the body organs are not sharply delimited .............................. Coccidian oocysts |
| B. | Protozoa detectable only under the compound microscope. |
| B.1. | Protozoa infecting the surface (skin, fins, nasal pits, or gills). |
| B.1.a | Protozoa that move. |
| B.1.a.1 | Cells up to 15 μm in size, possessing 2 flagella, moving with jerky, creeping motion or swimming spirally forward .............................. flagellates (Cryptobia, Ichthyobodo) |
| B.1.a.2 | Cells 20 μm and larger, either covered uniformly with cilia or with only several ciliary belts or circular ciliary wreaths. They move directly forward, glide over the surface, or roll on the spot .............................. Ciliates |
| B.1.a.3 | Cells with amoeboid movement and changes of body shape (about 20 μm in size) .............................. Amoebae |
| B.1.b | Sessile or motionless protozoa attached to the surface. |
| B.1.b.1 | Small transparent, pyriform cells not exceeding 15 μm in size .............................. Attached Ichthyobodo |
| B.1.b.2 | Pyriform or sac like cells, 30–300 μm in size, their cytoplasm yellowish or greenish and containing many refractile granules .............................. Parasitic dinoflagellates |
| B.1.b.3 | Rounded cells 40–60 μm in size, with rows of cilia; with a transparent cystic envelope .............................. Encysted ciliates (e.g., Amphileptus) |
| B.1.b.4 | Cells 40–100 μm in size, with cytoplasm dark due to refractile granules, and with bundles of tubules with knoblike ends protruding from their surface .............................. Suctorian ciliates |
| B.1.b.5 | Goblet-like or cylindrical cells of about 40–90 μm in length, each with a wide free end encircled by wreaths of beating cilia. The cells may contract a little .............................. Sessiline peritriches |
| B.1.b.6 | Protozoa of 40–60 μm in size, hidden within shells which often have an ornamental surface pattern .............................. Thecate amoebae |
| B.2. | Protozoa infecting the lumen of the intestine and/or the tissue of the intestinal wall. |
| B.2.a | Myxosporea (see A.2.a); Microsporidia (see A.2.b); coccidian oocysts (see A.2.d), which are as a rule encased with an amorphous mass of yellowish materia (yellow body); or amoebae .............................. (see B.1.a.3) |
| B.2.b | Cells up to 15 μm in size, with up to 8 flagella, moving about with a jerky motion or swimming directly forward .............................. Flagellates |
| B.2.c | Spindle-shaped cells, of about 30–140 μm in size, uniformly covered with cilia, with both ends pointed and with sluggish movement .............................. Protoopalina |
| B.2.d | Ciliated cells of another shape, up to about 120 μm in length .............................. Ciliates |
| B.3. | Protozoa infecting the urinary tract (from renal tubules of kidney to urinary bladder). |
| B.3.a | Plasmodial cells, 10 μm - 5 mm in size, with a variety of cell inclusions and surface outgrowths. .............................. Myxosporean trophozoites, mostly with spores (see A.2.a) |
| B.3.b | Microsporidia (see A.2.b) |
| B.3.c | Motile cells of 40–120 μm in size, with ciliary wreaths .............................. Endocommensal trichodinids |
| B.4. | Protozoa infecting the gall bladder Myxosporea (see B.3.A) and Microsporidia (see A.2.b) |
| B.5. | Protozoa infecting all tissues of the fish body .............................. Microsporidia (see A.2.b) Myxosporea (see B.3.a), or coccidians (see A.2.d). |
| B.6. | Protozoa infecting the blood. |
| B.6.a | Slender cells, typically 10–15 μm long, moving with a wriggling or undulating motion, with 1 or 2 flagella .............................. Kinetoplastid flagellates |
| B.6.b | Cells of about 3–15 μm in size, of amoeboid shape, displaying a twitching motion on the spot .............................. Developmental stages of some myxosporeans. |
3. Flagellates (Phylum Mastigophora)
Trophozoites move by means of one to many flagella. They have one nucleus, or exceptionally more monomorphic nuclei. Phylum includes free-living and many parasitic organisms of different groups (six classes), autotrophic forms with chloroplasts and heterotrophs without chloroplasts. They reproduce by binary fission.
Dinoflagellates grouped by botanists with algae and by zoologists with the protozoa represent large class of flagellates, 60% are free living and photosynthetizing, many are nonphotosynthetic or parasitic. Trophozoites have two unequal flagella, one transversal, within an equatorial groove, the other longitudinal within a longitudinal groove. Most dinoflagellates adapted to the parasitic way of life live as ectoparasites.
Piscinoodinium pillulare (Fig.7) is a dangerous ectoparasite in aquaria and fish cultures in tropical as well as in the temperate zone. It appears to be non-specific, infecting indiscriminately various fishes. Invades skin, fins and gills. The pyriform, sack-like or in grown state subspherical trophont is up to 160 μm long and appears yellowish-brown. After a period of growth of about 6 days at 25°C grown trophont drops of the host's surface, sinks to the bottom, rounds off, becomes a tomont and without secreting a common envelope, divides successively into 64 or 128 small cells. They divide again to produce 128 or 256 cells which differentiate into gymnospores. Pathogenicity is high. In heavily infected aquarium fish, infection symptoms are signs of discomfort, a golden, velvety hue on the body surface, spreading opercule, folding of fins and eventually emaciation.
Amyloodinium occelaltum is a marine counterpart of Piscinoodinium, Crepidoodinium cyprinodontum has been known thus far only from the gills of 3 species of cyprinodontid fishes. A dubious genus Oodinioides with a sole species O.vastator was described as an ectoparasite on the gills and skin of freshwater and marine fishes of various families.
The name “Oodinium” still survive among ichthyopathologists and aquarium hobbyists, although it is in fact the name of a different dinoflagellate genus ectoparasitic on marine invertebrates, not fishes.
T r y p a n o s o m a t i d s occuring in fish are currently assigned to the sole genus Trypanosoma, which includes 184 species in fishes. They have single flagellum which extends free or is attached to the body as the undulating membrane (its end extends free anteriorly) and a single, small disc-shaped kinetoplast. All are parasitic and are transmitted exlusively by leeches.
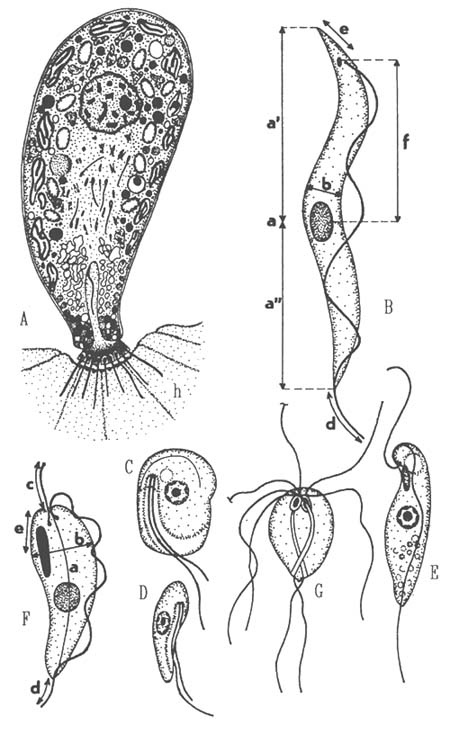
The life cycle in vertebrate host and vectors consist of a series of morphological changes comprising shifts in position of the kinetosomes and kinetoplast in relation to the nucleus and anterior end of the cell, it involves trypomastigote, epimastigote, sphaeromastigote and promastigote stages. Depending on the phase of infection at which the fish is examined, one finds young, slender or large adult forms only, or intermediate ones. A mix of forms is most likely to be result of sequential leech feeds. Trypanosomes make themselves apparent by their vigorous movement in fresh blood mounts, observed under the compound microscope at 100x magnification. If present, trypanosomes are found in Giemsa stained blood smears. The infections of extremely low intensity can be detected using hematocrit centrifuge techniques. At present about 100 named species of fish trypanosomes are defined and many of them may be synonymous. A due characterization of a fish trypanosome species existing or to be described, requires: 1) a detailed morphological analysis (shape, structure and position of the nucleus, shape and position of kinetoplast, subsurface striation in adult form, arrangement of the undulating membrane and free flagellum, cytoplasmic granules (Fig. 7); 2) morphometric evaluation; 3) ascertainment of pleomorphism in the course of infection; 4) observation on host specificity and 5) observation on the sequence of stages in the leech vector.
Ichthyobodo necator (syn. Costia necatrix), (Fig. 7) is a dangerous ectoparasite of practically all freshwater fish causing mortalities in young fish and fish with lowered resistance. The free non-feeding form has a flat, slightly asymmetrical, oval body, 10x5 μm; it is strongly convex dorsally and slightly concave ventrally. It swims in a hesitant, spiral way. A longitudinal groove traverse the hinder 2/3 of the ventral surface near its right margin. Two unequal flagella extend from the flagellar pocket. There is a small contractile vacuole close to the pocket, the cytostome opens at the border of the pocket. Centrally located nucleus, up to 2.5 μm has a large nucleolus. The feeding of attached form, fixed onto the epithelial cells, is highly modified. The cell furls in a pyriform shape, the flagella pointed off the fish surface. No digestive vacuoles are formed.
Cryptobia branchialis (Fig. 7) has been reported from the gills of an enormous number of hosts in Europe, Asia, North America and Africa. It abounds in organically polluted waters, feeding on bacteria and detritus particles. C. branchialis is drop-shaped, rounded anteriorly and tapering posteriorly, size range is 12–22×3.5–4.5 μm. At the side of the flagellar pocket is a contractile vacuole. The recurrent flagellum adheres to the cell surface along a wavy line. C. branchialis has been often found in enormous numbers on the gills of its freshwater hosts and acused of causing disease, but the adherence of the recurrent flagella inflicts no damage to the epithelial cells and reasons for the massive occurrence may be debilitated condition resulting in decreased repellent ability of gill surface.
Trypanoplasma species, especially T. salmositica, T. boreli and T. bullocki are known to cause disease and mortalities in many fish species, both feral and cultured ones. They have a wavy undulating membrane, much wider than in Cryptobia, which differ mainly in being transmitted by a leech and in undergoing a series of regular morphological changes during their life cycle. From the flagellar pocket at the anterior apex of the body extends a short anterior free flagellum and a long recurrent flagellum, which is attached to the body along its left, foldlike outstretched border with which it forms a striking undulating membrane. A single, large, elongated, densely staining kinetoplast extends from the apex of the cell where its pointed end subtends the flagellar kinetosome. The rounded nucleus lies opposite to the kinetoplast in the left border of the dorsolateral groove. During the progress of infection in the fish trypanoplasms manifest a marked pleomorphism, but unlike in fish trypanosomes it is difficult to characterize the shape differences in forms from early and late infection stages in more precise terms. The validity of many if not most species can be questioned due to great uniformity of alleged species. An adequate characterization of Trypanoplasma species requires inclusion of essentially similar features as those outlined for trypanosomes (Fig. 7). The best known species from freshwater fishes are: T. boreli, T. keysselitzy, T. tincae, T. carassii.
Hexamita salmonis (Fig. 7) is a common intestinal endocommensal of salmonids sometimes turning into a serious pathogen. The body is ellipsoidal or eggshaped, 7–14 x 3–10 μm. Ellipsoidal nuclei have large nucleoli. Length of flagella is one and half the body length. The flagellates swim quickly with slight wavering forward. The transmission occurs via cysts or trophozoites which may survive for some time in water. The cysts are ovoidal 10x7 μm, with the flagella divided inside in two. Although the parasite is quite common, the disease is rare in natural conditions and in cultured fish it only causes disease when the host's vitality is adversely affected by some predisposing factors.
Fig. 7: A - Piscinoodinium pillulare, cell organisation of a trophont attached to host cell (h); B - Measurements in Trypanosoma: a-body length (the actual length of the curved body, to be measured midway between the body sides, a'-midpoint of the nucleus to kinetoplast midpoint, a''-midpoint of the nucleus to the anterior body end (nuclear index), b-greatest body width, d-length of the free end of the flagellum, e-distance of the midpoint of the kinetoplast to the posterior end, f-midpoint of the nucleus to midpoint of the kinetoplast; C - Ichthyobodo necator, ventral view; D - lateral view; E - Cryptobia branchialis; F - Measurements in Trypanoplasma: a-body length, b-greatest body width, c-length of the anterior flagellum, d-length of the free end of the recurrent flagellum, e-distance of the midpoint of the kinetoplast to the anterior end; G - Hexamita salmonis.
4. Amoebae (Phylum Rhizopoda)
Amoebae are mostly difficult to determine. Their cell has a simple structure, the plasmalemma is naked or with an external test. Trophic stages move using pseudopodia or a simple protoplasmic flow. Flagella when present are usually restricted to developmental or sexual stages. The diagnostic features include primarily the locomotive form and behaviour, presence of flagellated stages, cyst structure and nuclear division patterns (stained cells are needed). There are very few specific endocommensals of fish, such as species of the genera Entamobea or Schizamobea, which under certain conditions can turn in true parasites. All other amoebae infecting fish are amphizoic species, i.e., free-living forms which can under certain conditions colonize the fish. Amphizoic species are found especially in families Hartmanellidae, Mayorellidae, Acanthamoebidae and Vahlkamphiidae (Fig. 8).
5. Coccidia (Phylum Apicomplexa)
Fish-infecting coccidia, members of the order Eimeriida are exclusively intracellular tissue parasites, located in 1the host tissue cell within a parasitophorous vacuole. Their life cycle comprises asexual phase, i.e., merogony, sexual phase, i.e., gamogony and sporogony. The latter produces the infective stages, oocysts, which contain sporocysts with sporozoites. The oocyst and sporocyst structure is of primary importance in distinguishing between genera and species and in practical determination.
Practical key for determination of fish-infecting coccidians
1. a) Oocyst with 4 “naked” sporozoites, without sporocysts,
which develops in submembrane position
.............................. Cryptosporidium
b) Oocyst with 2 sporocysts .............................. Isospora
c) Oocyst with 4 sporocysts .............................. 3
d) Oocyst with 8 sporocyst .............................. Octosporella (Fig. 8)
2. a) Sporocyst with 1,2 or many long knob-bearing
projections on their surface .............................. Calyptospora (Fig. 8)
b) Sporocyst without projections .............................. 3
3. a) Sporocyst with one pole bearing a special structure
(Stieda body) - pointed, collar - or knob-like .............................. 4
b) Sporocyst without a Stieda body, with wall composed of
2 valves adhering together along a longitudinal suture
line (which is sometimes difficult to see in the light
microscope .............................. Gousia (Fig. 8)
c) Sporocyst crystal-like, composed of 2 hexagonal valves
adhering along a transverse suture line
.............................. Crystallospora (Fig. 8)
4. a) Merogony and gamogony in submembrane position, sporogony
intracellular .............................. Epieimeria
b) Development as a rule intracellular, if not, so unlike
the above pattern .............................. Eimeria (Fig. 8)
6. Microsporidia (Phylum Microspora)
Microsporidia are widely distributed parasites of various teleosts in freshwater, estuarine and marine habitats. They may seriously endanger whole stocks of feral fishes as well as cultured ones. Microsporidia are strictly intracellular. In some species, the infected cell becomes hypertrophic (up to several mm) offering space to a mass of proliferating parasites, and is called the xenoma. The unicellular spores have an imperforate one-piece wall, contain one sporoplasm and an elaborate hatching apparatus where an extrudible hollow polar tube serves for the injection of the sporoplasm into the host cell (Fig. 8). Most microsporidian stages reach very small dimensions up to about 5 μm, which has always made their study rather difficult; plasmodial stages are up to 30 μm. The developmental cycle includes a proliferative phase (merogony or schizogony) producing a great number of parasites and sporogony which gives rise to mature spores (in most species of uniform shape, in some species macro- and microspores are produced (Fig. 8). In some genera mature spores are grouped together within an envelope (sporophorous vesicle or SPV), which originally covered the spore-producing (i.e., sporogonial) plasmodium. The diagnostic characters are spore structure, presence or absence of SPVs and diplokary, details of merogony and sporogony and structure of xenoma if present.
Practical key for determination of microsporidian genera
1. a) Spores are formed in SPVs localized directly in the
tissue; there is no xenoma (do not take for xenoma a
parasite focus encapsulated by connective tissue of
the host .............................. 2
b) Spores are formed in a xenoma, huge or small, appearing
mostly as small whitish nodules or corpuscles .............................. 4
2. a) SPVs free in the tissue .............................. 3
b) Clusters of SPVs within another envelope of parasite
origin, the sporophorocyst wall (not to be confused
with connective tissue cell capsule) .............................. Heterosporis
3. a) SPVs with a firm wall contain 8 spores .............................. Thelohania
b) SPVs with a firm wall contain 6 to about 200, mostly
many spores .............................. Pleistophora
4. a) The xenoma is a hypertrophic ganglion cell, spores are
of two kinds .............................. Spraguea
b) There are two kinds of xenomas: small compartmentalized
ones (up to 200 μm) and huge undivided ones (up to 4
mm) .............................. Ichthyosporidium
c) Xenomas of still another character .............................. 5
5. a) Xenomas form whitish nodules in the musculature,
consisting of numerous interlocked xenomas, spores with
a conspicuous inclusion in the posterior vacuole
.............................. Tetramicra
b) Xenomas stay single, spores without such an inclusion
.............................. 6
6. a) Dot-like xenomas, sporogonial plasmodia produce several
spores in direct contact with the host cell cytoplasm
.............................. 7
b) Dot-like or larger xenomas; one to many spores are
produced within membrane walled SPVs .............................. 8
7. a) Xenomas in the liver .............................. Microgemma
b) Xenomas in the gills and on the surface of visceral
organs .............................. Nosemoides
8. a) Dot-like xenomas in the gills or digestive tract; one to several spores in the SPV .............................. Loma
8. b) Dot-like to peas-sized xenomas subcutaneously and in various body organs .............................. Glugea
Fig. 8: A - Entamoeba ctenopharyngodoni - trophozoite; B - Hartmanella vermiformis - trophozoite; C - Vexillifera bacillipedes - trophozoite; D - Acanthamoeba polyphaga-cyst; E - Vahlkampfia debilis - cyst; F - Goussia carpelli; G - Calyptospora funduli; H - Crystallospora crystalloides; I - Octosporella notropis; J - Eimeria rutili; K - Diagrammatic representation of a microsporidian spore, e-exospore, en-endospore, c-cell membrane of the sporoplasm(s), n-nucleus, v-posterior vacuole, a-anchoring disc or polar sac; p-polaroplast, pt-polar tube; L - Microsporidian spores as seen in fresh state, a-Glugea destruens; b-Pleistophora elegans; c-microspore, d-medium spore, e-macrospore of P.priacanthicola; f-various spores of Glugea machari; g-spores of Microsporidium rhabdofilia
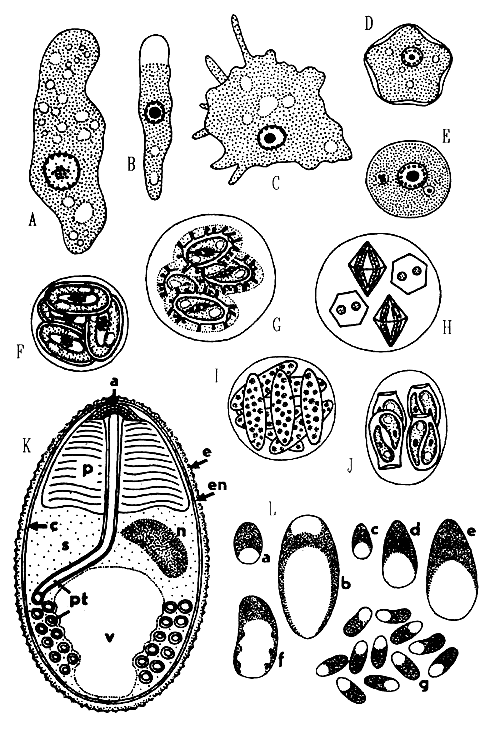
Within the genus, specific determination is also based on spore morphology, e.g., on their size and shape, on the size and shape of the large posterior vacuole and number of polar tube coils.
Among the microsporidians known for their pathogenic effect, is Pleisthophora hyphessobryconis pervading musculature of many species of ornamental fishes and inflicting high mortalities. P. oolytica develops within oocysts of Esox lucius and several other fish. P. ovariae is a serious pathogen in cultures of bait minnows in the USA (Notemigonus chrysoleucas). Glugea hertwigi elicits massive formation of large xenomas in the body of smelts of the genus Osmerus, resulting in mass fish kills.
7. Myxosporidia (Phylum Myxozoa)
Among the large number of myxosporeans there are many serious pathogens of commercially important fishes. They are parasites of organ cavities and tissues of fish. In salmonid cultures, heavy losses have been inflicted until recently by Myxosoma cerebralis and still are by Ceratomaxa shasta or by the enigmatic PKX (the agent of proliferative kidney disease). Myxosporidia are characterized by spores composed of several cell transfigured into 2 to 7 spore shell valves, 1 to 2 amoeboid infective germs (sporoplasms) and 2 to 7 polar capsules. The latter contain an extrudible filament of anchoring function. During the life cycle, some cells (the generative ones) are enclosed within others (somatic cells). Myxosporean spores may be of various shape and structure. Spore dimensions mostly fall within the range of 10 to 20 μm. The trophic (or vegetative) stages, or trophozoites, vary greatly in dimensions and shape. There are macroscopic plasmodia up to several mm in size, some species form small plasmodia only about 10 μm in size and produce a single spores. Since vegetative stages offer mostly no features important for classification, the taxonomy is based solely on the shape and structure of the spore.
Practical key for determination of myxosporean genera (PC - polar capsule; exclusively marine species are marked with an asterisk)
1. a) Mature spore contains only one PC .............................. 2
b) Two PCs per spore (one of them can be much smaller than the other one) .............................. 7
c) Three PCs per spore .............................. *Trilospora
d) Four PCs per spore .............................. 24
e) Five PCs per spore .............................. *Pentacapsula
f) Six PCs per spore .............................. *Hexacapsula
g) Seven PCs per spore .............................. *Septemcapsula
2. a) Spores pyriform, drop-like or elongated ellipsoidal
.............................. 3
b) Spores are more or less spherical, with the sutural
line difficult to see .............................. 6
c) Spores have a rounded ellipsoidal body with a large,
sac-like and curved extension .............................. *Auerbachia
3. a) The suture line of the two shell valves is sigmoidal
.............................. *Coccomyxa
b) The suture line of the two shell valves is straight
.............................. 4
4. a) Spores without a bifurcated caudal process .............................. 5
b) Spores with a bifurcated caudal process .............................. Phlogospora
5. a) Spores with PC discharging apically and axially
.............................. Thelohanellus
b) Spores with PC discharging subapically and to the side.
.............................. Neothelohanellus
6. a) Spores with three shell valves (two small ones and one
large) difficult to discern in light microscope;
exclusively histozoic .............................. *Unicapsula
b) Spores with two shell valves adhering along a delicate
sinuous suture line (exclusively coelozoic)
.............................. *Globospora
7. a) PCs located each separately in the ends of a spindle
-shaped or elongated spore .............................. 8
b) PCs not terminally in the opposing ends, but also not
close to each other .............................. 10
c) PCs close to each other .............................. 16
8. a) The polar filament within the capsule is a thin
spirally round tube of about the same thickness all
its length through .............................. 9
b) Polar filament strongly tapers from its base to the
tip; within the PC it does not form a regular coil,
being rather folded and bent over several times ..............................
.............................. *Sphaeromyxa
9. a) Spores have as a rule more or less pointed ends
.............................. Myxidium
b) Spores are mostly ellipsoidal, with rounded or bluntly
pointed ends and almost spherical PCs .............................. Zschokkella
10. a) Elongated spores with central, transversal sutural
line; PCs near opposite ends discharge laterally ................................... *Fabespora
b) Rounded or ovoid spores with PCs set widely apart in
the sutural plane .............................. 11
c) Spherical, rounded or pyramidal spores have PCs in a
plane perpendicular to the sutural line which is
mostly sinuous .......................... 12
11. a) Spores spherical or subspherical; in marine fishes
.............................. *Ortholinea
b) Spores ovoid; flattened parallel to the sutural
plane; in freshwater fishes .............................. *Neomyxobolus
12. a) Shell valves without projections .............................. 13
b) Shell valves with various projections .............................. 14
13. a) Spores spherical of subspherical .............................. *Sinuolinea
b) Spores inversely pyramidal or triangular and with
rounded outlines .............................. *Myxoproteus
14. a) Spores spherical, each shell valves bears in about its
center a hollow, mostly horn-like projection .............................................. *Davisia
b) Spores inversely pyramidal with tapering end extending
backwards; at the anterior surface of each valve, a
wing-like projection is attached .............................. *Bipteria
see also .............................. *Paramyxoproteus
c) In addition to valvular projections, a stiff, keel-like
membrane runs sidewise along the sutural line .............................. 15
15. a) Valvular projections wing-like as in Bipteria
.............................. *Neobipteria
b) Valvular projections keel-like, meridionally along
midline of the valves .............................. *Schulmania
c) Valvular projections in form of thickenings covering
the spore apex and slightly unstuck off its surface .............................. *Noblea
16. a) Spores elongated, asymmetrical, curved and very
thin-walled .............................. Parvicapsula
b) Spores essentially bilaterally symmetrical .............................. 17
17. a) The two spherical PCs are tandem in position and at
distance from the anterior end; two fine projections
at both posterior and anterior end of the spindle
shaped spore .............................. Neohenneguya
b) PCs are set in plane essentially perpendicular to
the sutural plane .............................. 22
18. a) Large spherical PCs in the center of the spore which
is triangular in sutural view .............................. Wardia
b) PCs more or less close to the anterior apex of the
spore .............................. 19
19. a) Spores without any projections or veils .............................. 20
b) Spores with projections or veils .............................. 21
20. a) Spores spherical or almost spherical .............................. Sphaerospora
b) Spores elongated in the direction perpendicular to the
sutural plane, sometimes arcuate; the depth of the
shell valve may reach the dimensions of the sutural
diameter .............................. Leptotheca
c) Spores crescent-shaped, extremely elongated in the
direction perpendicular to the suture line
.............................. Ceratomyxa
21. a) Subspherical spores are enveloped in a large
membranaceous veil .............................. *Palliatus
b) Triangular spores are laterally drawn into wing-like
membranous projections .............................. *Alatospora
c) Same as above, but the projections are doubled to
parachute-like pockets .............................. *Pseudoalatospora
d) Spores spindle-shaped, with a pair of long posterior
projections .............................. *Myxobilatus
e) Spores spherical to bullet-like with numerous thread- or
pin-like projections .............................. Hoferellus
22. a) Spores without projections .............................. 23
b) Spores with projections .............................. 24
23. a) The sutural line straight .............................. Myxobolus
b) The sutural line strongly sinuous .............................. Spirosuturia
24. a) A single caudal projection .............................. Unicauda
b) Two caudal, often slightly divergent projections
.............................. Henneguya
c) Two caudal, projections extend in opposite directions
Dicauda
d) Four posterolateral projections .............................. Tetrauronema
e) Two projections extend laterally from one side of the
posterior end of the spore .............................. Laterocaudata
f) The valves of broadly triangular spores are drawn
into filamentous processes on each side, each pair
being connected by a filament .............................. Trigonosporus
25. a) Four shell valves .............................. Kudoa
b) Two shell valves .............................. 26
26. a) Spores spherical .............................. Chloromyxum
b) Spores almost spherical, with one or two caudal
projections .............................. Caudomyxum
c) Spores spindle-shaped, with two caudal projections
.............................. Agarella
d) Spores elongated, asymmetrical, curved and thin-walled
.............................. Neoparvicapsula
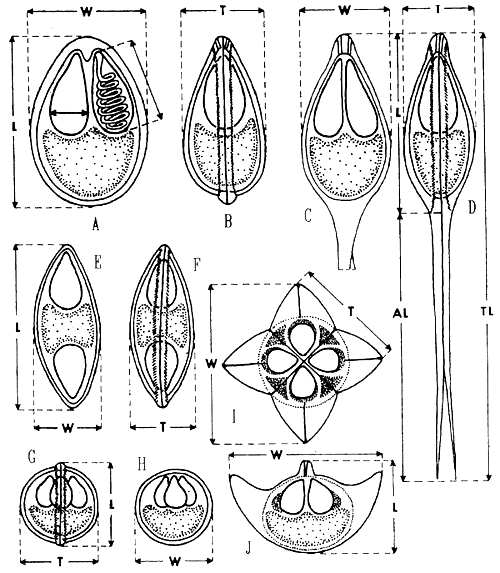
Presently, there are about 1100 myxosporean species described from fish. Unfortunately, in many descriptions, the spores have very often been quite inadequately characterized. For the proper description of myxosporean species fresh spores have to be observed. To make an accurate diagnosis we have to take measurements according to diagram in Fig. 9. In spores with polar capsules situated at one end, the length (L) is the distance between the apex and the posterior (opposite) end. In Bipolaria, it is the distance between the two opposite ends of the spore. Perpendicular to the length, there is the width (W) which is measured in the plane of the suture, and the thickness (T) measured in the plane perpendicular to the sutural plane of the spore. There is the guideline for description of new myxosporean species by Lom 1989 (see references).
Some of the most serious pathogens of commercially important fishes are:
Myxobolus cerebralis (syn. Myxosoma cerebralis) is the agent of the “whirling disease” of salmonid fry affecting head and vertebral column cartilage. Spores are extremely variable, oval to circular in front view (L 7.4–9.7 × W 7–10 × T 6.2 – 7.4 μm). The development up to mature spores takes about 2 month at 19°C. Depending on water temperature, clinical signs (“black tail”) appear 2 to 8 weeks after exposure, abnormal tail behaviour, the “whirling” appears later, 2 to 3 month p.i. Chronic infection may be manifested in the second year by cachexia, listlessness and head deformation. Head and gill arch cartilage are sites of predilection. Light infections can be revealed by recovery of spores after the host tissue has been digested. Trypsinization may be preceded by decalcification.
Sphaerospora renicola, the best known species of the genus Sphaerospora is widely distributed in intensive cultures of Cyprinus carpio (Europe, Israel). Disporic pseudoplasmodia are located in the lumen of renal tubules, spores are subspherical (L 7.3 x W 7.2 μm), with ovoid polar capsules (PC) (2.8 x 2.3 μm) and 4 filament turns oriented perpendicularly to the PC. S. renicola may be a serious pathogen because of extrasporogonic developmental sequences which elicit among others swimbladder inflammation in one-year-old carp in its 3–4 month of age.
Fig. 9: Methods of measurement of myxosporean spores of various genera: A and B - Myxobolus in frontal and side sutural view; C and D - Henneguya in frontal and side view; E and F - Myxidium in frontal and side view; G and H Chloromyxum in side or sutural (G) and frontal (H) view; I - Kudoa in apical view; J - Kudoa in one of the possible side views which is the diagonal one. Measurement of the polar capsule is indicated in A: L - length of the spore, W - width of the spore, T - thickness of the spore; in spores with caudal appendages such as Henneguya, Al - length of the caudal appendage, Tl - total length of the spore.
“P K X” is the abbreviation for the still undetermined agent of proliferative kidney disease (“P K D”) of salmonid fingerlings. The agent is a myxosporean trophozoite (not specific for its salmonid hosts) which elicits an intense defence reaction of the host, the intersticial nephritis. Annual losses in the UK alone were estimated at 640 000 £ in the early eighties.
Ceratomyxa shasta is a dangerous pathogen of North American west-coast salmonids causing serious losses in cultured and wild salmonid populations. C. shasta infects all layers of the entire digestive tract wall, but can also be disseminated in other organs. Small disporic pseudoplasmodia area 13 x 19 μm in size, spores (L 6–8 x W 14–17 μm) are strongly arched, with broadly rounded ends, smooth valves and PC 2.2 μm in diameter. The mode of infection is still obscure. Exposure to contaminated bottom sediments or to contaminated water induces infection.
8. Ciliates (Phylum Ciliophora)
Ciliates are highly organized protists with the cell covered by cilia arranged in rows or kineties. In some groups the number of cilia is reduced or they may be secondarily absent. Ciliates show nuclear dualism, having as a rule generative diploid micronuclei and vegetative polyploid macronuclei. The buccal apparatus serves for ingestion of particular food. Only several groups of ciliates became secondarily astome, feeding by pinocytosis. They divide by transverse binary fission, sexual process is conjugation.
Ciliates living in and/or on fishes range from completely harmless ectocommensals (e.g., Erastophrya) to most noxious parasites as e.g., Ichthyophthirius or Tetrahymena. Manifestations of their pathogenic action are most varied at the tissue and organ levels. The mechanisms of the damage are irritation of the surface cells in extremely heavy growth of ectocommensals and surface cell destruction, penetration into deep tissue layers and ingestion of the cell debris thus produced in e.g., Tetrahymena or Ichthyophthirius infections. Gill and skin tissue destruction is then necessarily reflected by the debilitated or moribund condition of the fish. Ectoparasitic ciliates include species that are the most common parasites of fishes (Trichodina). Although in free water trichodinids are common and form sometimes dense populations, morbidity due to Trichodina is rather an exception. In fish confined to ponds, tanks and aquaria, trichodiniasis is a frequent problem. Much more so, the ciliates Chilodonella and Ichthyophthirius are rather rare in fish in free waters, but require a lot of attention to prevent losses in freshwater fish culture, especially in young fish. Their determinal economic impact is enhanced by their lack of host specificity and cosmopolitan distribution.
For a proper identification all important ciliate features must be observed in live as well as stained specimens. Thus in addition to fresh mounts, slides stained with nuclear stains and impregnated with Klein's method or with protargol are necessary.
Genus Balantidium includes 10 species of endocommensal ciliates with cystosome at the base of anteriorly located vestibulum. Their body is uniformly covered with ciliary rows. They are endocommensals which can turn into histophagous parasites capable of abrading the epithelium, penetrating into the subepithelial intestinal layer and feeding on the tissue cells. Balantidia destroy the epithelium, cause desqúamation, extensive ulceration, and when deep in the tissue, they elicit granuloma formation. The losses caused by B. ctenopharyngodoni (Fig. 10) were recorded in 1 to 3 year old grass carp (Ctenopharyngodon idella).
Chilodonella piscicola (Fig. 10) is one of the most dangerous ectoparasites which lives on gills and skin of practically all freshwater fish and also in estuarine and brackish waters. The body is asymmetrically oval, with a notch in the posterior margin, 55 x 43 μm (range 30–80 x 20–62 μm). In the right ciliary band there are mostly 8 to 11 kineties, in the left ciliary band, 12 to 13.
Chilodonella hexasticha differs from the preceeding in the absence of a notch at the posterior body margin, in less numerous and more loosely spaced kineties (Fig. 10) and in smaller body size (30–65 x 20–50 μm). Its biology and pathogenicity are the same. It appears that C. piscicola tends to infect rather the fingerlings while C. hexasticha prevails on older fish. Both species may occur on the same host.
Chilodonellas seem to be of a great ecological adaptability, proliferating both in cold and warm water. Under conditions which favour their proliferation, chilodonellas may cover the body surface in a contiguous layer. They disintegrate the surface tissue by means of their oral cytoskeletal armament and feed on the cell debris. Pathological manifestations may vary depending on the intensity of infection. Gill disintegration and necrosis renders the gills nonfunctional, fish lose osmotic balance and die.
Ichthyophthirius multifiliis (Fig. 10) is a commonly distributed dangerous ectoparasite in freshwater fish culture, known as an agent of “white spot disease”. It belongs to Ophryoglenina, a suborder of large ciliates with polymorphic life cycle. Their buccal cavity opens at the bottom of a deep depression called prebuccal area, which is densely covered with arched anterior ends of kineties of the right body side. Close to the buccal cavity lies a lens-like refractile organelle. The life cycle of I. multifiliis comprises a small migratory stage in search of a host, the theront (Fig. 10); the feeding and growing stage in the skin or gills of fish, is the trophont (Fig. 10) with a characteristic, large horse shoe-shaped macronucleus. After it has reached a certain size it escapes from the host and encysts on a convenient substrate as a tomont. Within its cyst, it divides by a series of 10 to 11 divisions to produce small tomites. They break through the cyst wall to become theronts again. The duration of the life cycle stages, their size and number depend on the ambient temperature. During the period of growth in the host's tissue, the trophont reaches the size up to 1 mm, increasing its volume about 3000 times. Due to the growing trophont volume the microcirculatory disorders and associated necrotic changes are enhanced. I. multiphiliis feeds on the cell debris, forms small cavities beneath the epithelial layer. Both in gills and skin the regressive changes of the epithelium are the most deleterial. Economic importance of ichthyophthiriasis is manifested not solely by direct losses. In fish culture, it is primarily the reduced increment.
P e r i t r i c h s are ciliates characterized by inverted bell-shaped, conical or cylindrical body with the ciliature encircling the apical pole. The antapical pole is equipped with holdfast organelles, a scopula or adhesive disc. Somatic ciliature is reduced.
S e s s i l i n a, which include genera Ambiphrya, Apiosoma, Riboscyphidia, Epistylis, Propyxidium, Vorticella, Zoothamnium and Carchesium are attached as a rule permanently to the substrate with specialized area called scopula. The scopula either directly adheres to the substrate often being cemented with a sticky substance, or it secretes a stalk. The stalk may bear one ciliate or if branched, it may bear many ciliates sometimes forming colonies. For a reliable description of sessiline peritrichs, observation of living, non-fixed ciliates is absolutely necessary, in order to record the true body shape, as well as morphology of the peristomial region. The body of peritrichous ciliates is generally uniform and all structural peculiarities are helpful in characterization. The morphology of the stalk, scopula and telotrochs (migratory stages) is most important. Essentially, sessiline peritrichs found in fish are ectocommensals or symphorionts that use their hosts as a living moving substrate to settle where they gain access to a convenient source of food particles - organic debris and water-born bacteria. They are specifically adapted to the life on the surface of certain species of fishes.
M o b i l i n a are constantly moving peritrichous ciliates which can attach temporarily to the substrate by means of a slightly concave adhesive disc reinforced by a system of skeletal elements of proteinaceous character. The disc can contract and its function is reminiscent of a sucker. They do not form cysts, the transmission is direct by swimming ciliates. Fish are invaded by members of a single family - Trichodinidae. All trichodinids are essentially commensals, five genera occur in fish. Ectozoic species use their host as a convenient substrate upon which they glide and to which they temporarily attach. They feed on waterborne particles and bacteria as well as detritus particles from the fish surface. Trichodinids never occur in larger amounts on a fish in good health condition. In a fish debilitated by some other factors or on young fry, the trichodinids can massively proliferate, and then they behave like serious parasites.
Fig. 10 - Balantidium ctenopharyngodoni; A - Chilodonella piscicola, ventral side; B - the cyst of C. piscicola; C - kineties on the ventral side of C. hexasticha; D - Ichthyophthirius multifiliis, a grown trophont in ventral view; E - I. multifiliis, a theront in ventral view; F - Diagnostic features of trichodinid ciliates: skeletal parts of the adhesive disc, s - rods in the border membrane, r- -radial pins, nu - number of radial pins per denticle, d - denticles, dd - diameter of the denticulate ring, da - diameter of the adhesive disc; G - horse-shoe shaped macronucleus of trichodinids with three possible positions of micronucleus; H - a denticle of Trichodina: t - length of thorn, c - width of the central part, b - length of blade, e - length of denticle; I - shape of denticles in Trichodinella.
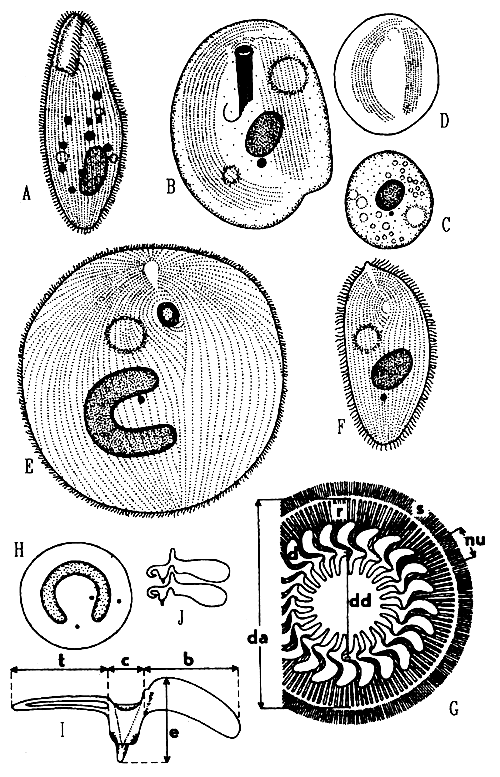
The taxonomy of trichodinids is based on the structure of the buccal ciliature and on the appearance of the adhesive disc and numbers and size of its constituents. All these features can be revealed only by the silver impregnation technique of Klein.
Key to the genera occuring in fish:
1.a) The adoral ciliary spiral makes two and half to three
turns .............................. Vauchomia
b) The adoral spiral makes one turn of slightly less or
more (330° to 540°) .............................. 2
c) The adoral spiral makes one half to three quarters of a
turn (an arch of 150° to 290°) .............................. 3
2.a) The denticles have well developed both thorns and
blades .............................. Trichodina
b) The blades of denticles are stunted .............................. Hemitrichodina
3.a) The denticles have well developed thorns .............................. 4
b) The thorns are stunted to form short crooks or
platelets .............................. Trichodinella
c) No thorns at all, central part indistinct, blades
triangular .............................. Dipartiella
4.a) The centrifugal projections of denticles (blades) are
attached to the central part almost perpendicularly and
the denticles are interlocked only by their central
conical parts .............................. Paratrichodina
b) Blades extend from the central part obliquely
backwards; denticles are interlocked by central parts
and by anterior projections of blades fitting into
corresponding notches in blades of the preceding
denticles .............................. Tripartiella
For specific determination, the appearance of structures revealed by Klein's technique is most important, as well as the measurements of constituents of the adhesive disc (see Fig. 10). Size of denticles is measured according to Fig. 10. The diameter of the macronuclear horse-shoe is measured after Fig. 10. In Trichodinella, the thorn is reduced to a short crook.
Recommended literature
Arthur J.R., Lom J. (1984): Trichodinid protozoa (Ciliophora: Peritricha) from freshwater fishes of Rybinsk Reservoir, USSR. J. Protozool. 31, 82–91.
Canning E.U., Lom J. (1986): The Microsporidia of vertebrates. Academic Press, pp. 289. Dyková I., Lom J. (1983): Fish Coccidia: An annotated list of described species. Folia Parasitol. 30, 193–208.
Halliday M.M. (1976): The biology of Myxosoma cerebralis: the causative organism of whirling disease of salmonids. J. Fish Biol. 9, 339–357.
Lom J. (1979): Biology of the trypanosomes and trypanoplasms of fish. In: Lumsden W.H.R. and Evans D.A. (Eds.). Biology of the Kinetoplastida, Academic Press, London - New York - San Francisco, 269–337.
Lom J. (1981): Fish invading Dinoflagellates: a synopsis of existing and newly proposed genera. Folia Parasitol. 28, 3–11.
Lom J., Arthur J.R. (1989): A guideline for the preparation of species descriptions in Myxosporea. J.Fish Dis. 12, 151–156.
Mitchell L.G. (1977): Myxosporida. In: J.F.Kreier (Ed.), Parasitic protozoa, Vol. 4, 115–154. Academic Press.
Page F.C. (1976): An illustrated key to freshwater and soil amoebae. Freshwater Biological Assoc. Publ. No 34, Titus Wilson and Son, Kendal, England, pp. 155.
Popovský J., Pfiester L.A. (1990): Dinophyceae (Dinoflagellida). In: Süsswasserflora von Mitteleuropa. Band 6. Gustav Fisher Verlag, Jena, Stuttgart, pp. 272.
Reichenbach-Klinke H.H. (1980): Krankheiten und Schädigungen der Fische. Gustav Fisher Verlag, Stuttgart, New York, pp. 472.
Roberts R.J. (Ed.) (1978): Fish pathology. Bailliere Tindall. London, pp. 318.
Scheubel J. (1973): Die sessilen Ciliaten unserer Süsswasserfischen unter besonderer Berücksichtigung der Gattung Apiosoma Blanchard. Zool. Jb. Syst. 100, 1–63.
Upton S.J., Reduker D.W., Current W.L., Duszynski D.W. (1984): Taxonomy of North American fish Eimeriidae. NOAA Technical Report NMFS 11, (U.S. Department of Commerce, National Marine Fisheries Service), pp. 18.
B) PARASITIC METAZOA
(F. Moravec, R. Ergens, V. Našincová, T. Scholz)
In addition to protozoans, an important role among the agents of fish diseases is played by multicellular or metazoan parasites. These belong to several very different groups that include enormous numbers of species occurring in fishes both as ectoparasites and endoparasites, attacking practically all organs and tissues of the host's body. In view of a considerable morphological diversity of metazoans it is by far impossible to deal with these parasites in more detail in this very short review and, therefore, their correct identification will require the use of special literature. Only the most important groups of metazoans parasitic in fishes have been included.
The body size of metazoan parasites may be very different, ranging from less than one millimetre up to several tens of centrimetres and, therefore, most of them can be observed with the naked eye. Nevertheless, the use of binocular microscope for fish examinations is quite necessary. Live and/or freshly caught (dead) fish are preferable. In general, the detailed examination of a small number of fish (10–15 of each species) is sufficient to recognize the parasitological situation in the locality. Following the external examination of the body surface, fins, gills and nasal cavity, the fish body is dissected and individual organs and tissues (e.g. digestive tube, liver, urinary bladder, kidneys, gall-bladder, swim-bladder, mesenteries, eyes, brain, pieces of muscles) are thoroughly examined for the presence of endoparasites.
In general, external parasites can be kept in clean freshwater for a few hours without serious damage, but all internal macro-parasites should be kept in saline and fixed within a few minutes to one hour. The most commonly used fixatives for parasites are 70 % ethanol or 2–4 % formalin. Trematodes and cestodes are usually fixed slightly pressed between a glass slide and a coverslip. Delicate organisms and semi-microscopic forms (e.g. some monogeneans) need special techniques to reduce damage and prevent loss. There are many special procedures of preparing the parasites for their subsequent microscopical examination. For example, the trematodes and cestodes are usually stained with carmine, nematodes cleared in glycerine or lactophenol, etc.
The metazoan parasites of fishes belong mostly to organisms loosely referred to as worms (Platyhelminthes, Nematoda, Acanthocephala, Hirudinea), less often to crustaceans and molluscs.
Platyhelminthes - flatworms
They tend to be flat, although many of them are fusiform or even filiform. They may be segmented or unsegmented and they all are equipped with characteristic attachment organs.
Key to classes of Platyhelminthes
1 Ectoparasites; one posterior attachment organ, with one or more pairs of median hooks and varying numbers of marginal hooks and/or clamps .............................. Monogenea
- Endoparasites; attachment organs not as above .............................. 2
2 One attachment organ, armed with hooks and/or suckers (some without this organ). Gut absent. Body ribbon-like, segmented or unsegmented .............................. Cestoda
- Two sucker-like attachment organs. Gut present, usually bifurcated. Body unsegmented, flat to fusiform …Trematoda
Class Monogenea
The monogeneans (Fig. 11) are hermaphroditic flatworms parasitic mostly on or in fishes and some other aquatic or amphibious cold-blooded vertebrates, occasionally on aquatic invertebrates. They are most commonly found in the gill chamber, on skin, in buccopharyngeal cavity, or in other organs communicating directly or indirectly with the exterior (nostrils, cloaca, urinary bladder, etc.). Their development is without intermediate hosts. The chief attachment organ (opisthaptor) is posterior, more or less discoid, muscular, provided with paired or unpaired anchors and a number of marginal hooks or with muscular suckers or clamps without or with supporting sclerites and anchor complexes. Accesory adhesive organs may be present in form of armed plaques, lappets or appendices.
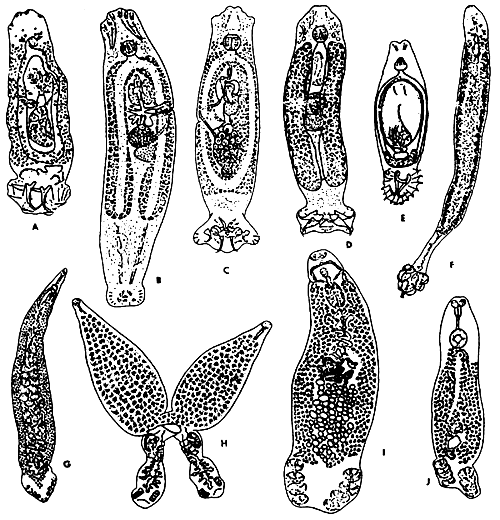
Fig. 11: The representatives of some families of the Monogenea. A - Dactylogyridae; B,C - Ancyrocephalidae; D - Tetraonchidae; E - Gyrodactylidae; F - Diclobothriidae; G - Mazocraeidae; H - Diplozoidae; I - Discocotylidae; J - Octomacridae. (After Gussev and Bychowsky, 1957).
Anterior adhesive organ (prohaptor) may be represented by so-called head organs, which are made up of distended cephalic gland ducts opening along the lateral margins of the anterior extremity, occasionally by glandular structures often accompanied by paired or unpaired suckers, paired pseudosuckers or sucking grooves.
Almost all the previous authors, including the most recent ones, have placed great emphasis on the external morphology, particularly on the sclerotized parts of the body such as opisthaptoral anchors, clamp sclerites, copulatory organ, etc. These hard parts are, of course, of special value as taxonomic criteria. The internal anatomy, particularly that of the gonads, is also of great taxonomic value, although not much attention has been paid to it by the majority of helminthologists. The class Monogenea is usually divided into two subclasses.
Key to subclasses of Monogenea
1 Opisthaptor with 1–3 pairs of anchors and with 7–8 pairs of marginal hooks, without clamps ..............................Polyonchoinea
- Larval opisthaptor mostly with 5 pairs of marginal hooks. Opisthaptor of the adult is developed as suckers or clamps supported by sclerites or a combination of clamps and anchor complexes ..............................Oligonchoinea
The most frequent Polyonchoinea infecting freshwater fishes are represented by numerous genera and species of 4 families (3 orders).
Key to orders and families of Polyonchoinea
1 (6) Oviparous
2 (5) Opisthaptor with 7 pairs of marginal hooks and with 1–2 pairs of anchors ..............................Order Dactylogiridea
3 (4) Opisthaptor with 7 pairs of marginal hooks and with 1 pair of anchors ..............................Family Dactylogiridae
4 (3) Opisthaptor with 7 pairs of marginal hooks and with 2 pairs of anchors ..............................Family Ancyrocephalidae
5 (2) Opisthaptor with 8 pairs of marginal hooks and with 2 pairs of anchors .............................. Order Tetraonchidea Family Tetraonchidae
6 (1) Viviparous ..............................Order Gyrodactylidea Family Gyrodactylidae
The most frequent Oligonchoinea infecting freshwater fishes are represented by some genera and species of 5 families (2 orders).
Key to orders and families of Oligonchoinea
1 (2) Opisthaptor with 3 pairs of sessile suckers, each provided with a large curved sclerite ending in a hook and a prominent opisthaptoral appendix bearing 3 pairs of anchors..............................Order Diclobothriidea Family Diclobothriidae
2 (1) Opisthaptor with 4 or more pairs of clamps and with 1–2 pairs of anchors on terminal lappet..............................Order Mazocraeidea
3 (4) Opisthaptor with 4 pairs of clamps and with 2 pairs of anchors..............................Family Mazocraeidae
4 (3) Opisthaptor with 4 pairs of clamps and with 1 pair of anchors.
5 (6) Two adults permanently fused in form of letter..............................Family Diplozoidae
6 (5) Each adult exists individually
7 (8) Testes numerous, gill parasites of Salmonidae..............................Family Discocotylidae
8 (7) Testis one, gill parasites of Cyprinidae..............................Family Octomacridae
Class Cestoda
Tapeworms (Fig. 12) represent a platyhelminth group with exclusively endoparasitic pattern of life. They mostly parasitize in the intestine of vertebrates. Their extreme adaptations to endoparasitism resulted in complete loss of digestive system and an increase in reproductive capacity that often staggers the imagination. A total of 4.000 species of cestodes have been described; many of them are parasitic in fishes either as adults or as larvae.
Although considerable variation of morphology occurs between different orders of tapeworms, there are underlying similarities that unite the orders into the class Cestoda.
The tapeworm body usually consists of a chain of segments called proglottids, each of which contains one or more sets of reproductive organs. The proglottids are continuously produced by a process of asexual budding. The entire body formed by proglottids is called a strobila, and a segmented strobila is said to be polyzoic. In some groups, particularly in fish cestodes, the body consists of a single segment, and is then said to be monozoic. At the anterior end a holdfast organ or scolex is usually found, that is the principal means of locomotion of these animals. Depending on the group, the scolex may be provided with suckers, grooves, hooks, spines, glandular areas, or combination of these.
All fish cestodes are hermaphroditic and their life cycle includes at least one intermediate host, mostly invertebrates (oligochaetes, copepods, amphipods, etc.).
Key to orders of fish cestodes
1 (2) Strobila with no internal segmentation; one set of reproductive organs present ..............................Caryophyllidea
2 (1) Strobila with internal segmentation; more than one set of reproductice organs present.
3 (4) Scolex with no true suckers, bothria, bothridia, or tentacles; no external segmentation ..............................Spathebotriidea
4 (3) Scolex with one of the holdfast types listed above (3); external segmentation usually distinct.
5 (6) Scolex with two bothria ..............................Pseudophyllidea
6 (5) Bothria absent; scolex armed with four rounded suckers ..............................Proteocephalidea
Short survey of the orders of fish cestodes with brief characterization of some important representatives.
Caryophyllidea
Tapeworms with nonsegmented body containing only one set of reproductive organs. The scolex is simple, has shallow groowes or loculi, shallow suckers, or is frilled. It is never armed with hooks. Life cycle of all species involves a freshwater oligochaete as the intermediate host. Caryophyllideans are parasites of freshwater teleosts, especially Cypriniformes (mainly in Palaearctic Eurasia and America) and Siluriformes (in Asia).
Caryophyllaeus fimbriceps is an important parasite of carp (Cyprinus carpio) cultures in eastern Europe and the USSR. This species, about 15 mm long, is typified by the shape of scolex armed with deep grooves. As a consequence of introduction of the species Khavia sinensis, another carp parasite, to many European countries, C. fimbriceps is now rather rare and does not represent a veterinary problem. Some other representatives of the genus Caryophyllaeus have been recorded in siluroid fishes in India and southeast Asia (Java).
Khavia sinensis is larger (length of body up to 120 mm) than C. fimbriceps and its scolex bears deeper incisions forming festoon-like shape of scolex. It occurred originally only in east Asia. Owing to the import of herbivorous fishes, particularly of grass carp (Ctenopharyngodon idella, it has been spread to many European countries and most parts of the USSR.
Several species of the genus Lytocestus have been hitherto described from catfishes, mainly Clarias batrachus, in India, southeast Asia and Africa. Members of this genus are characteristic by the presence of undifferentiated scolex and the absence of vitelline follicles in postovarian zone.
Caryophyllidean cestodes with scolex often constricted off from body and parasitizing siluroid fishes in Africa, mostly those of the genus Synodontis, belong to the genus Wenionia.
Spathebothriidea
Parasites of teleost fishes, both marine and freshwater. Cestodes bearing undifferentiated, funnel-shaped or hollow, cup-like organ. External segmentation is absent, but there are several serially arranged sets of male and female reproductive systems.
Cyathocephalus truncatus, a common parasite of salmonid and some other freshwater fishes in Europe, the USSR and North America, has scolex with funnel-shaped apical adhesive organ. Strobila flat, with 20 to 45 sets of reproductive organs. Intermediate hosts are amphipods.
Pseudophyllidea
Members of this large order are parasites of fish, amphibians, reptiles, birds, and mammals. Their scolex has dorsal and ventral depressions called bothria, which are quite agile and serve as holdfast and locomotor organs. The life cycle involves a crustacean first intermediate host and mostly fish second intermediate host.
Larval stages called plerocercoids of Ligula intestinalis, up to several tens of centimetres long, are common in many freshwater fishes, particularly cyprinids. Plerocercoids, localized in body cavity, are rather pathogenic for their fish hosts and can cause even their death.
Representatives of the genus Diphyllobothrium are cosmopolitan parasites of reptiles, marine and terrestrial mammals, and birds. Scolex has narrow and deep bothria, and distinct neck. Plerocercoids of some species, including the species D. latum, parasitizing humans, are commonly found in flesh of coregonid, perciform, and other freshwater fishes.
In Asian teleosts, members of the genus Senga are often recorded. They are typified by the rectangular scolex, with bilobed apical disc bearing many small hooks on margins. Eggs are anoperculate.
The species Bothriocephalus acheilognathi is a dangerous parasite of carp in fish culture, imported from Far East to many countries in Europe, America and Africa. Scolex of this tapeworm is elongate, with apical disc and narrow, very deep bothria. External metamerism complete, length of strobila up to 15 cm. Uterine pores median. Copepods serve as the first and the only intermediate hosts of the parasite.
Triaenophorus nodulosus and T. crassus are common, circumboreal parasites of pike (Esox spp.). Plerocercoids are parasitic in internal organs and muscles of many species of freshwater fishes, mainly perciforms and salmonids. Adults and larvae from fishes are characteristic of the presence of four trident-shaped hooks on scolex and two shallow botria.
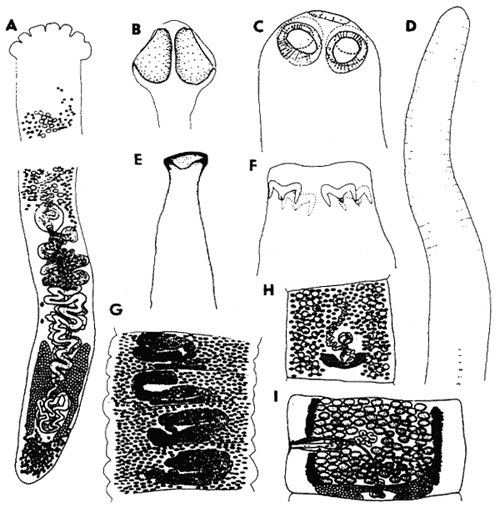
Fig. 12: A - Khawia sinensis (order Caryophyllidea), total view; B, G - Bothriocephalus acheilognathi (Pseudophyllidea), scolex and gravid segment; C, I - Proteocephalus neglectus (Proteocephalidea), scolex and mature segment; D - Ligula intestinalis, plerocercoid (Pseudophyllidea), anterior end; E - Cyathocephalus truncatus (Spathebothriidea), scolex; F - Triaenophorus nodulosus (Pseudophyllidea), scolex; H - Bothriocephalus claviceps (Pseudophyllidea), mature segment.
Proteocephalidea
Members of this order are easily recognized by the presence of a scolex with simple suckers and follicular, usually lateral vitellaria. Most of them are parasites of freshwater fishes.
Tapeworms of the genus Gangesia have rounded scolex with rostellum armed with one or two circles of hooks. They parasitize on siluroid fishes in Europe and Asia.
The genus Proteocephalus comprises many parasites of freshwater fishes, amphibians and reptiles. Scolex unarmed, with four simple suckers, sometimes with fifth apical organ. P. ambloplitis is commonly found in many species of freshwater fishes in North America; P. neglectus is a veterinary important parasite of trout in Europe; P. exiguus parasitizes coregonid fishes in Europe and Asia.
Class Trematoda
Fish trematodes (Fig. 13) are endoparasitic. They possess bilaterally symmetrical, dorsoventrally flattened, unsegmented bodies, usually oval or lanceolate. The majority of trematodes fall into the range of 1–30 mm. Trematodes are commonly equipped with two attachment organs, the oral sucker at or near the body's anterior end and the ventral sucker, or acetabulum, the position of which varies from near-anterior to near-posterior, with all position in between. Some species lack one of suckers or even both. Most fish trematodes are hermaphroditic.
The life cycles of trematoda involve more than one (often three) hosts and include several morphologically different developmental stages. The first intermediate hosts are usually molluscs, which release motile larval stages - cercariae. Freshwater fish may serve as the second intermediate hosts of the next developmental stages (metacercariae) or as definitive hosts. Metacercariae are encysted in various inner organs and tissues, adults are localized mostly in the alimentary system, less often in excretory or blood-vascular systems and in body cavities.
The trematode fauna of freshwater fish is diverse. Moreover, the taxonomic treatment of the class Trematoda has been frequently reassessed as new information becomes available, particularly that concerned with life cycles and larval stages. The most complete information on fish trematodes gives Yamaguti (1971). Some more important representatives parasitizing freshwater fish, listed according to families, are mentioned below.
ADULT TREMATODES
Bucephalidae
Gasterostomatous trematodes without oral sucker, with rhynchus as adhesive organ at anterior end. Pharynx and oesophagus present. Intestine simple, saccular; no acetabulum. Members of the genera Busephalus, Bucephalopsis, Rhipidocotyle, Prosorhynchus are common parasites of freshwater fish.
Sanguinicolidae
Trematodes of lanceolate body shape, living in blood-vascular system; usually without suckers. Pharynx absent, intestine X- or H-shaped. Thin-shelled eggs, without operculum, sometimes with lateral projection (Sanguinicola). Some species can cause serious damage to fish in aquaculture.
Monorchiidae
Parasites of marine, sometimes freshwater, fish. Trematodes from freshwater fish are small, about 1 mm in length, with elongate body covered with spines. Acetabulum in anterior half of body. Genital pore submarginal. Caeca short, testis double (Paleorchis), or caeca long and only one testis present (Asymphylodora).
Gorgoderidae
Trematodes with smooth body divided into a narrow forebody and large hindbody. Suckers well-developed, pharynx absent, caeca reaching near to posterior extremity. Fish gorgoderids, belonging mainly to the genus Phyllodistomum, are localized in urinary bladder.
Allocreadiidae
Nearly exclusively parasites in digestive tract of freshwater fish. Trematodes with elongate, mostly unarmed body. Oral sucker without appendages (Allocreadium, Orientocreadium) or with typical muscular appendages (Bunodera, Bunoderina, Crepidostomum). Acetabulum in anterior half of body. Digestive system well-developed; caeca long, terminating near posterior extremity.
Opecoelidae
Trematodes resembling in adult morphology members of the family Allocreadiidae, with small body, less or more elongate, usually unarmed. Parasitic in digestive tract of marine and freshwater fish (Nicolla, Helicometra, Sphaerostomum, Plagioporus).
LARVAL STAGES (metacercariae)
Bucephalidae
Metacercariae of several genera (Bucephalus, Bucephalopsis, Rhipidocotyle, Prosorhynchus) are commonly found encysted on gills, in skin, fins, eyes, brain, subcuticular tissue, and muscles of freshwater fish. Metacercariae are morphologically similar to adults, parasitizing predatory fish.
Diplostomatidae
Body divided into two parts; forebody leaf-shaped, hindbody considerably smaller. Oral sucker small, terminal, anterior end provided sometimes with two pseudosuckers. Acetabulum postequatorial, holdfast organ situated in posterior part of forebody. Metacercariae motile, unencysted, localized in eyes (Diplostomum, Tylodelphys), or encystated in brain, skin, muscles and other organs of many freshwater fish (Neodiplostomum, Posthodiplostomum). Some representatives (Diplostomum spp.) are serious pathogens causing even death of highly infected fish.
Strigeidae
Metacercariae of tetracotyle type are encysted in musculature, body cavity and on surface of various inner organs. Cysts oval to pear-shaped. Suckers well-developed, pseudosuckers usually present. Holdfast organ postacetabular. Important genera parasitizing fish are Apharyngostrigea, Cotylurus and Apatemon.
Clinostomatidae
Free or encysted metacercariae of the genera Clinostomum and Euclinistomum are localized in body cavity, muscles or inner organs of fish. Body elongated, with both suckers in anterior third of body. Pharynx absent. Caeca reaching near to posterior extremity, usually with lateral diverticula.
Opisthorchidae
Metacercariae elongate, encysted in muscles of mainly cyprinid fish. Suckers relatively large, acetabulum near middle of body. Caeca long, reaching near posterior extremity. Large excretory bladder filled with numerous granules. Adults of some species (Opisthorchis felineus, O. viverrini, Clonorchis sinensis) are important human liver parasites. Man acquires infection by eating raw fish flesh with encysted metacercariae.
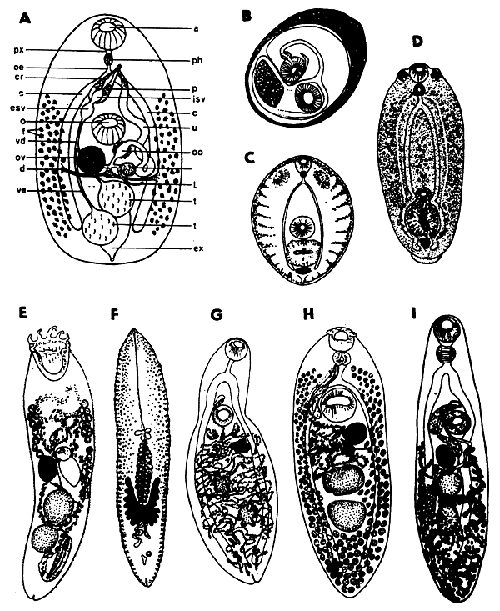
Fig. 13: A - A generalized trematode: a - acetabulum, c - caecum, cr - cirrus, d - vitelline ducts, esv - external seminal vesicle, ex - excretory vesicle, f - vitelline follicles, isv - internal seminal vesicle, L - Laure s canal, o - oral sucker, oe - oesophagus, oo - ootype, ov - ovary, p - prostate, ph - pharynx, px - prepharynx, sr - seminal receptable, t - testis, u - uterus, vd - vas deferens, ve - vas efferens; B-D - Metacercariae: B - Opisthorchis viverrini, C - Cotylurus pileatus, D - Diplostomum sp.; E-I - Adult trematodes: E - Bucephalus polymorphus, F - Sanguinicola inermis, G - Phyllodistomum elongatum, H - Crepidostomum farionis, I - Orientocreadium batrachoides.
Heterophyidae
Encysted metacercariae with two poorly developed suckers. Oral sucker provided sometimes with spines (Centrocestus, Phagicola, Pygidiopsis); acetabulum often modified and armed with numerous spines and sclerites (Heterophyes, Metagonimus, Haplorchis, Stellantchasmus). Metacercariae encysted mostly in muscles, sometimes in gills, fins or under scales of many freshwater fish. Adults of some species have been found in man.
Nematoda - roundworms
The nematodes (Fig. 14) represent a large group of metazoan invertebrate animals. They are noted for the elongate, cylindrical body covered with strong protecting cuticle that usually has fine transverse striations. The mouth opening is usually surrounded by lips and cephalic papillae, sometimes a sclerotized buccal capsule occurs. The digestive tube is formed by the oesophagus, often subdivided in two distinct parts, the intestine and the rectum. The sexes are separated. The male tail is sometimes provided with caudal alae, genital papillae and usually one or two sclerotized spicules are present. They are free-living or parasitic in animals and plants. From about 16,000 described species, about 40 % are animal parasites.
Nematodes belong to the most frequent parasites of freshwater fishes, some of them being the agents of serious fish diseases. In addition to adult forms, some nematode species occur in fishes only as larvae. The definitive hosts of these species are then various piscivorous vertebrates (fishes, reptiles, birds, mammals), whereas the fish harbouring these larvae serves as the intermediate or the paratenic host. The development of fish nematodes is either direct or, more frequently, with the participance of an obligate intermediate host (usually an invertebrate).
Key to subclasses of Nematoda parasitic in vertebrates
1 Eggs with plug at either pole; males with one spicule or spicule absent; oesophagus cylindrical or with oesophageal glands free in pseudocoel stichosome; phasmids absent.............................. ..............................Adenophorea
- Eggs without polar plugs, rarely operculate at one pole, or hatching in uterus; males usually with two spicules, exceptionally spicule one or absent; oesophagus never in form of stichosoma; plasmids present ..............................Secernentea
Adenophorea
are represented in fishes only by one order Enoplida; only its superfamily Trichuroidea includes adults from fishes, while Dioctophymatoidea contains some genera (Dioctophyma, Eustrongylides) parasitic in fishes only as larvae.
Key to families of Trichuroidea
1 Complete digestive tract present, posterior region of female cylindrical ..............................Capillariidae
- Digestive tract incomplete; posterior region of female expanded to form vesicle ..............................Cystoopsidae
Pseudocapillaria salvelini is a frequent intestinal parasite of salmonids and cottids in the Holarctic Region. Intermediate or paratenic hosts are various oligochaetes. Schulmanela petruschewskii is parasitic in the liver of many species of freshwater fishes of different families in Europe. It is a serious parasite of pond-reared carp and rainbow trout. Oligochaetes serve as intermediate hosts. Cystoopsis acipenseris is parasitic in cysts under the skin of sturgeons in Palaearctic Eurasia and North America. Its intermediate hosts are gammarids.
Secernentea
include 3 orders containing the species parasitizing freshwater fishes.
Key to orders of Secernentea
1 Oesophagus with bulb; male with reduced caudal papillae; generally only one spicule; body short and stout.............................. ..............................Oxyurida (Only a few species are known to occur in freshwater fishes in tropical Asia and South America).
- Nematodes lacking most of the above characters ..............................2
2 Anterior extremity triradiate (except in some Seuratoidea); head end with three lips or lips absent; oesophagus not divided into muscular and glandular parts.............................. ..............................Ascaridida
- Anterior extremity bilaterally symmetrical; head end with two lateral lips or lips reduced or absent; oesophagus divided into anterior muscular and posterior glandular parts..............................Spirurida
Key to superfamilies of Ascarida from freshwater fishes
1 Head end with well developed lips, sometimes separated by interlabia; oesophagus simple and cylindrical or terminated by ventriculus; precloacal sucker absent in male; genital papillae numerous ..............................Ascaridoidea
- Lips on head strongly reduced or absent; oesophagus simple and cylindrical, or divided into two parts having or not having same diameter; ventriculus absent; precloacal sucker may be present in male; genital papillae not numerous ..............................Seuratoidea
Superfamily Ascaridoidea
Raphidascaris acus in noted for the presence of the posterior ventricular appendix. Adults are parasitic in the gut of pike and some other predatory fishes in the Holarctic Region. Larvae occur encysted in many other fish species. Hysterothylacium bidentatum is an intestinal parasite of sturgeons of Palaearctic Eurasia. It possesses the posterior venticular appendix and the anterior intestinal caecum. Goezia ascaroides is parasitic in the stomach of European catfish. Its cuticle is armed with transverse rows of minute spines.
Superfamily Seuratoidea
Paraguimperia tenerrima of the family Quimperiidae is an intestinal parasite of European eels. Other congeneric species occur in eels in India and North America. Cucullanus truttae of the family Cucullanidae is a frequent intestinal parasite of salmonids of the Holarctic Region. Its intermediate hosts are lampreys. Many congeneric species occur in various fishes in other regions.
Key to superfamilies of Spirurida from freshwater fishes
1 Pseudolabia always absent; oesophagus divided into muscular and glandular portions or muscular throughout … 2
- Head end with pseudolabia, sometimes rudimentary; muscular and glandular portions of oesophagus well differentiated ..............................3
2 Buccal capsule well developed, orange-brown; parasites of digestive tract ..............................Camallanoidea
- Buccal capsule reduced or absent (except for Anguicolla), colourless. Not parasitic in digestive tract ..............................Dracunculoidea
3 Caudal alae in male absent. Pseudolabia almost absent .............................. ..............................Thelazioidea
- Caudal alae in male present. Pseudolabia present..............................4
4 Pseudolabia small, not covering entire cephalic surface. Collarette absent ..............................Habronematoidea
- Pseudolabia large, massive. Body cuticle immediately behind pseudolabia often expanded to form collarette.............................. ..............................Physalopteroidea
Superfamily Camallanoidea
Camallanus lacustris is noted for a complex orange-brown capsule formed by two lateral valves and provided with two large tridents. It is a frequent intestinal parasite of many fish species (mainly percids) in Europe. Intermediate hosts are copepods.Procamallanus spiculogubernaculus with a simple buccal capsule is a common parasite of some catfishes in India.
Superfamily Dracunculoidea
Philometra ovata is a common parasite of some cyprinids in Europe. Large females occur in the abdominal cavity, whereas small males are found on the swimbladder. It develops through copepods.
Fig. 14: Nematoda. A, B - nematode morphology, generalized and diagramatic (A - male, B - female; abbreviations: a - anus, bc - buccal capsule, co - cloacal opening, cp - caudal papillae, go - glandular oesophagus, i - intestine, mo - muscular oesophagus, nr - nerve ring, o - ovary, s - spicules, t - testis, u - uterus with eggs, v - vagina, vu - vulva); C-P - anterior and posterior body ends (C, D - Pseudocapillaria, D, K, O - Raphidascaris, E, L, P - Spinitectus, F - Camallanus, G, H - Anguillicola, I - Philometra, J, M - Skrjabillanus).
Skrjabillanus tincae is parasitic in mesenteries and kidneys of tench in Europe. These are very delicate, thin and long nematodes; its intermediate hosts are branchiurids. Anguillicola crassus is a danger parasite of the swimbladder of eel which was recently introduced into Europe from East Asia. Its intermediate hosts are copepods and ostracods.
Superfamily Thelazoidea
Rhabdochona denudata of the family Rhabdochonidae is a frequent intestinal parasite of some European cyprinids. Its intermediate hosts are mayflies.
Superfamily Habronematoidea
Cystidicola farionis of the family Cystidicolidae is a pathogenic parasite of the swimbladder of various Holarctic salmonids. Its intermediate hosts are gammarids. Spinitectus inermis of the same family is parasitic in the stomach of European eels. Its body is provided with rings of cuticular spines. Many congeneric species occur in fishes in other continents. Intermediate hosts are various crustaceans and aquatic insects.
Superfamily Physalopteroidea
Pseudoproleptus notopteri of the family Physalopteridae is parasitic in the stomach of the featherback, Notopterus notopterus, in India.
Acanthocephala - spiny-headed worms
Acanthocephalans (Fig. 15) belong to one class and they are all parasitic. The body shows three externally recognizable regions: the trunk, the neck and the retractable proboscis armed with a set of posteriorly pointing hooks the number, size and arrangement of which are of taxonomic importance. The proboscis functions to anchor the worm in place more or less permanently, by penetrating the host's intestinal wall. The trunk is a sac-like structure, subcylindrical, usually with pseudosegmentation. The digestive tract is absent. The sexes are separate. Their development involves an invertebrate intermediate host.
Many acanthocephalan species of different genera and families are parasitic in both marine and freshwater fishes all over the world. The forms parasitizing freshwater fishes belong to 3 orders.
Key to orders of freshwater fish acanthocephalans
1 Cement glands syncytial; cuticle nuclei giant..............................2
- Cement glands in form of several compact formations; cuticle nuclei usually small..............................Palaeacanthocephala
2 Trunk with cuticular spines..............................Gyracanthocephala
- Trunk without cuticular spines..............................Neoacanthocephala
Order Gyracanthocephala
Pallisentis ophiocephali belonging to the family Quadrigyridae parasitizes mainly fishes of the family Channidae in tropical Asia. It develops probably through copepods.
Order Neoacanthocephala
Neoechinorhynchus rutili of the family Neoechinorhynchidae is parasitic in many fish species of different families (mainly cyprinids) in Palaearctic Eurasia. Its development is through ostracods.
Order Palaeacanthocephala
Acanthocephalus anguillae of the family Echinorhynchidae is a frequent parasite of cyprinids and some other fishes in Europe. Its intermediate host is a freshwater isopod Asellus aquaticus.
Pomphorhynchus laevis of the family Pomphorhynchidae is a common parasite of cyprinids and other fishes in Europe. Its intermediate hosts are various species of freshwater gammarids.
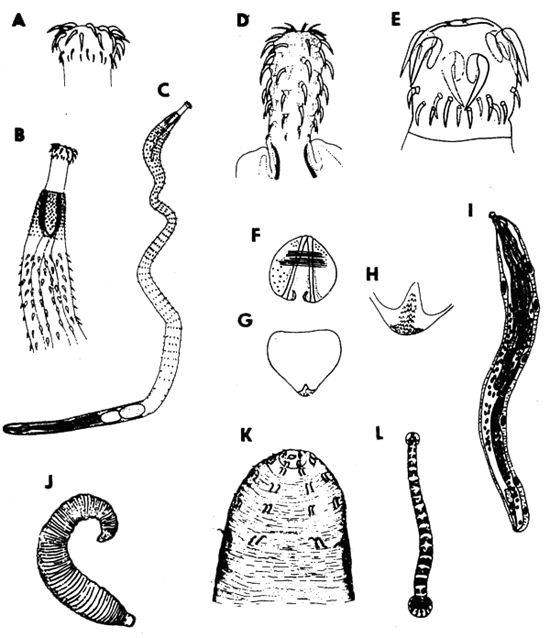
Fig. 15: A-E, I - Acanthocephala (A-C - Pallisentis, male; D - Acanthocephalus, E, I - Neoechinorhynchus); F-H - Mollusca (glochidium of Unio); J-L - Hirudinea (J, K - Acanthobdella, L - Piscicola).
Crustacea - crustaceans
Species parasitic on fishes are found in higher as well as in lower classes of Crustacea (Fig. 16). They occasionally undergo structural modifications of various degree called forth by the adaptation to parasitism which involve fusion (partial or complete) of segments of various parts of the body and reduction and change in the limbs. In addition a progressive development of attachment organs, simplifications of external morphology (development of various hornlike, rootlike and lobed processes, etc.) occur. This review will take in turn three major divisions of crustaceans.
Key to major crustacean groups
1 Entire dorsal surface of body divided into many narrow segments, tagmata poorly developed; parasite immovably attached to external surface, buccal or branchial cavity of fish..............................Isopoda
- Dorsal surface of body with reduced segmentation and well developed tagmata, or unsegmented.............................. 2
2 Pair of compound eyes; body covered anteriorly with dorsal shield; main attachment organs suckers of modified first maxillae; parasite capable of movement over host surface..............................Branchiura
- Compound eyes absent; double median eye present or absent; shape of body varied; parasites anchored and sessile, attached more or less firmly to surface on fish, or able to move over its surface..............................Copepoda
Copepoda
Undoubtedly the largest group of Crustacea parasitic on fishes, Copepoda have among them species showing various stages of adaptation to parasitism. At one end of the adaptive range are those ectoparasites whose structure has not yet been greatly affected by their association with the host. Some of them are freely mobile, able to swim and to change one fish host for another. At the other end of the range there are completely sessile endoparasites, sometimes with greatly reduced appendages and shapeless, sac-like bodies.
There are known some 1,700 species of copepods parasitic on fishes. A large proportion of them belongs to the suborder Siphonostomatoidea (75%), some 20% to Poecilostomatoidea, and only about 5% to Cyclopoida. The members of the first two major taxa are predominantly marine, whereas those of the third one are exclusively freshwater. Copepods infest the skin, gills, nasal cavities, buccal cavities, and the fins of infected fishes. Their development is mostly straight, without a change of hosts. Massive infestation with copepods, particularly with Ergasilus, Lernaea and Salmincola often cause fish epizootics and mortality.
The copepods parasitizing freshwater fishes belong to three suborders, distinguishable from one another mainly by their mouth parts.
Key to suborders
1 Mouth forming short, subcylindrical tube, sometimes situated at apex of more or less prominent cone; mandible rod-shaped, with flat distal part usually, though not invariably, bearing row of teeth..............................Siphonostomatoidea
- Mouth and mandible not as above..............................2
2 Mouth not forming tube, more or less gaping; mandible falcate..............................Poecilostomatoidea
- Mouth not forming tube, more or less gaping; mandible small, with unciform, unarmed distal segment..............................Cyclopoida
Suborder Cyclopoida
This suborder contains many species parasitic in fishes; practically all species are freshwater. Most of them belong to the family Lernaeidae; it is rather evenly divided into two groups of species, the first of them not overly modified and still retaining most, or at least some, of their segmentation, the second highly metamorphosed and usually possessed of a more or less well developed holdfast as an attachment organ.
Lamproglena pulchella and some 20 other congeneric species are parasitic on the gills of freshwater teleosts in the old World. The segmentation of their body is partly retained. The above species is a frequent parasite of many species of cyprinids and pike in Europe and Central Asia.
Lernea cyprinacea is a representative of the genus including about 40 species parasitizing freshwater teleosts in Europe, Africa and North and South Americas. The metamorphosed female has a diminutive, semispherical cephalothorax followed by a well developed holdfast consisting of simple or dividing branches. Males are not parasitic. The anterior part of the parasitic female is embedded in musculature of the fish, its posterior part is protruding. Penetrating deep into the tissues of the fish and developing within them a large holdfast, it causes extensive tissue damage and has been known to bring about mass kills of valuable fish stocks.
Suborder Poecilostomatoida
This suborder contains many species and genera representing a very wide range of adaptations to parasitic life, beginning with forms which are only slightly modified from the ancestral free-living condition, and ending with forms strongly metamorphosed under the impact of parasitism. Both ectoparasites and endoparasites are found among them. Most of them are small and tend to be more or less mobile and less prone to cause extensive tissue damage.
Ergasilus sieboldi is a widely distributed parasite of gills of many European freshwater teleosts, causing frequently problems in some pond-reared fishes, particularly tench. It belongs to the family Ergasilidae distributed in various continents. Only the female is parasitic. The entire life cycle and the process of mating occurs in water, before the females find and settle on their hosts.
Suborder Siphonostomatoida
It comprises a significant majority of copepods parasitic on fishes, infecting them in both freshwater and marine habitats. Their general diagnosis is very difficult because of the great morphological variety. Salmincola salmoneus is a member of the family Lernaeopodidae comprising about 250 species distributed in the fresh waters in the northern hemisphere and in the oceans. The genus Salmincola contains some 17 species parasitic on salmonids and their relatives. The above species is parasitic on the gills of salmons.
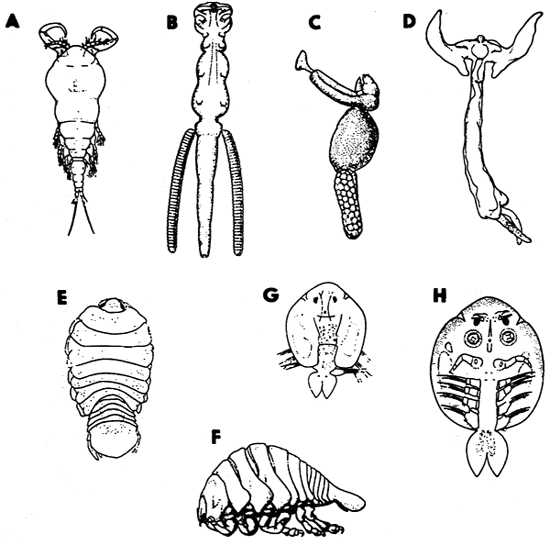
Fig. 16: Crustacea. A - Ergasilus sieboldi, B - Lamproglena pulchella, C - Salmincola salmonea, D - Lernaea cyprinacea, E, F - Ichthyoxenus amurensis, G, H - Argulus coregoni.
Branchiura
A rather small group, comprising fewer than 150 species grouped in 5 genera; as many as 100 of them belong to the genus Argulus which has a world-wide distribution, with species in both marine and freshwater habitats. They are particularly important in freshwater aquaculture, where their harmful impact upon fish can assume devastating proportions. Although unequivocally parasitic, they are capable or spending prolonged periods without a host. They mate free-swimming and deposit eggs in characteristic clusters on various submerged objects. They are also capable of moving from fish to fish. Most species are only loosely specific and may be found on the skin and gills of many fresh water fish species. Argulus foliaceus is one of the most distributed species of the genus, occurring in Europe, Palaearctic Asia and some other continents, parasitizing there many fish species.
Isopoda
Parasitic isopods found on fishes all belong to the suborder Flabellifera and are found through the world, virtually in all habitats, throughout by far the greatest number are marine. They are well adapted to their parasitic way of life and can cause considerable damage to the host tissues at the infection site. They are important parasites in certain freshwater environments of South America and Africa, where they infect fishes used locally for food.
Isopods associated with the external surfaces of fish sometimes produce gall-like depressions in the skin and muscles of the body wall. Others live in the buccal or branchial cavities.
Ichthyoxenus amurensis penetrates the skin, entering the body cavity in the region of the pectoral fins of some freshwater cyprinids in the Far East. It feeds on blood.
Hirudinea - leeches
Leeches (Fig. 15) are annelid worms with more or less uniformly metameric bodies. Only about a quarter of leech species are parasitic, their parasitism being of a temporary, intermittent character. Although they vary quite widely in their morphology, they are always large enough to be observed with the naked eye, narrowly oval or lanceolate in outline, usually more or less dorsoventrally flattened and provided with anterior and posterior suckers; the latter is always larger than the former and fairly regularly discoid. The body is always transversely striated, the grooves dividing it into very definite annuli, three of which usually correspond to a single segment. The most important anatomical feature of leeches is their alimentary canal whose capacity is enlarged by numerous diverticula; their number and arrangement are important taxonomic characters. Unfortunately, they often require serial sectioning for accurate examination. Leeches from fishes have been reported as feeding on host's blood, body fluids or ingesting tissue particles.
Key to subclasses of Hirudinea
1 Anterior end of body with ventral bristles. Anterior sucker absent or if present, its posterior lip weakly developed..............................Acanthobdelliones
- Anterior end of body without ventral bristles. Anterior sucker with well developed posterior lip..............................Hirudiniones
Subclass Acanthobdelliones
This subclass is represented only by two species, Acanthobdella peledina and Paracanthobdella livanowi, belonging to two monotypic families differing from each other mainly by the presence or absence of the anterior sucker. Both species are parasitic on the skin of various salmonids of the Palaearctic Asia and, in the first species, also of Europe.
Subclass Hirudiniones
This subclass is represented by some 400 species of which members of the families Piscicolidae and Glossiphonidae, including several genera, are parasitic on fishes.
Piscicola geometra with the usual body length 15–30 mm is a widespread freshwater parasite of many fish species of different families and orders of the Palaearctic Region. It is usually found on the body surface, fins and sometimes in buccal and branchial cavities.
Cystobranchus mamillatus is, as the foregoing species, a member of Piscicolidae. It is a specific parasite of burbot of the Palaearctic Region; it is found in the branchial cavity and on gills.
Placobdella sp. of the family Glossiphonidae has been reported from some freshwater fishes (Anabas, Channa, Puntius, Tilapia, Trichogaster) from Thailand. Congeneric species occur only on freshwater fish (but also on aquatic birds, reptiles and amphibians).
Mollusca - molluscs
The freshwater bivalve moluscs of the family Unionidae, during their early development, pass through a larval form known as the glochidium (Fig. 15). Hatching out from eggs incubated between the gill lamellae of the parental individual, glochidia are expelled into the surrounding water, where they can survive for a limited period of time. Glochidia are miniature bivalves, the margins of their shells equipped with sharp teeth; a filamentous tentacle is situated between the valves. To survive, the glochidium, which is often narrowly host specific, must find a fish. Glochidia become encysted on gills, fins and skin of the fish where the young molluscs undergo extensive development and the definitive shell replaces the early larval valves. Upon maturation, which takes three weeks to several months depending on the water temperature and certain other conditions, they fall to the substrate as young mussels.
If the fish gills are attacked by a very large number of glochidia, the fish may suffer from respiratory difficulties. The escape of glochidia from their cysts leaves open wounds that are subject to fungal or microbial infections.
For identification, glochidia should be removed from fish gills, fins and skin without any squashing or deformation of their shape, because size and shape are distinctive characters of species. The size of glochidia of a given species of mussel may vary with the habitat; the glochidia infesting river and lake fishes tend to be slightly larger than those infesting small stream fishes. However, since the pre-parasitic and parasitic stages of many species of glochidia are nearly equal in size, the identification made can thus be ascertained by comparing the size of glochidia from gravid mussels of known species collected from the same area of study.
Recommended literature
Anderson R.C., Chabaud A.G., Willmott S. (eds.) (1974–1983): CIH Keys to the nematode parasites of vertebrates. Nos. 1–10. Commonwealth Agricult. Bureaux, Farnham Royal, Bucks, England.
Bauer O.N. (ed.) (1985): Key to parasites of freshwater fishes of the USSR fauna, Vol. 2. Parasitic Metazoa, Pt. 1. Nauka, Leningrad, pp. 424. (In Russian.)
Bauer O.N. (ed.), 1987: Key to parasites of freshwater fishes of the USSR fauna, Vol. 3. Parasitic Metazoa, Pt. 2. Nauka, Leningrad, 583 pp. (In Russian).
Crompton D.W.T., Nickol B.B., (1985): Biology of the Acanthocephala. Cambridge Univ. Press, Cambridge, London, New York, New Rochelle, Melbourne, Sydney, pp. 519.
Fernando C.H., Furtado J.I., Gussev A.V., Hanek G., Kakonge S.A., (1972): Methods for the study of freshwater fish parasites. University of Waterloo, Canada, Biology Series No. 12, 76 pp.
Margolis L., Kabata Z. (eds.) (1984–1989): Guide to the parasites of fishes of Canada, Pts. i, II, III. Can. Spec. Publ. Fish. Aquat. Sci. 74, 101–107.
Yamaguti S. (1971): Synopsis of digenetic trematodes of vertebrates. Part I, II. Keigaku Publishing Co., Tokyo, pp. 1074 + 349 Plts.