(Z. Svobodová, B. Vykusová)
Analysis of the peripheral blood of fish serves for diagnostic purposes; apart from this main purpose, it is also used at present to examine the effect of toxic substances on the fish, to evaluate the condition of the fish, to evaluate the non-specific resistance of different fish breeds and strains and of the brood fish, to assess the suitability of feeds and feed mixture pellets, to evaluate the effect of stress situations etc. The recommended methods described here include only well-tested and well-proven procedures and techniques of the determination of the different haematological parameters. The values of the different haematological parameters are significantly influenced by endogenous and exogenous factors, so it is not easy to determine their physiological range. Hence, the haematological and biochemical data given in the different chapters should only serve for rough orientation.
Blood sampling
Blood is sampled for ichthyohaematological examination as soon as the fish are taken out of the environment in which they have lived. The main criteria for selection a particular method of sampling include the size of the fish, the amount of blood needed and the further fate of the fish caught for different examinations.
1/ Blood sampling in fish fry
Blood from fry at an individual weight of at least 8 grammes can be sampled by the methods of cardiopunction, using a glass capillary long about 200 mm whose inner surface is lined with a fine film of heparin before use. Lift the fish, fixed head down, to the level of the eyes and apply the tip of the capillary at an angle of about 60° (in relation to the longitudinal axis of the fish body) about 1-2 mm cranially from the mid point, which is the point of intersection of the longitudinal axis of the body with the line connecting the cranial edges of the base of both pectoral fins. At this point the carp fry have the so-called stigma: a shallow, usually pigmented depression in the skin up to 1 mm wide. Now drive the tip of the heparinized blood-collecting capillary quickly through the body wall to the pericardium and further to the heart (Fig. 21). Blood, appearing in the capillary, is the most reliable evidence that one of the cavities of the heart has been hit. When the collection is finished, pull the tip of the capillary out of the wound, hold the capillary in horizontal position and then turn it upside down several times to let the blood mix well with the anticoagulant mixture.
In heavier fish fry, for example at a weight of about 20 g, the blood collecting capillary may be replaced by a dry and clean heparinized injection needle. Such needles are driven into the heart in the same way as the glass capillary. The cardiopunction technique can also be used with older fish: this is so in those cases when the fish can be killed.
2/ Collecting blood from fish weighing above 200 g, including brood fish
In these fish, the best method is that of collecting blood by punction of the caudal blood vessels (Fig. 22). On the caudal peduncle ventral side the unpaired dermal scale is removed in caudal direction from the anal fin base. Within the central plane, about 1 cm caudally from the anal fin, a sufficiently long needle is introduced, firmly held on the cone of a heparinized disposable syringe, into the fish body in a craniodorsal direction at an angle of 45°. The described method is fully recommendable, mainly in larger series of blood collection; collection of 2 ml of blood from cyprinids weighing above 1000 g involves no risk of loss of the treated specimens.
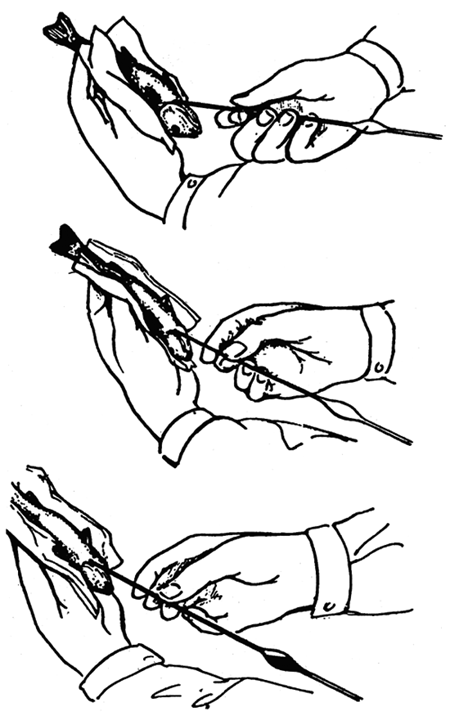
Fig 21: Blood sampling in fish fry by the method of cardiopunction using a 200 long glass capillary.
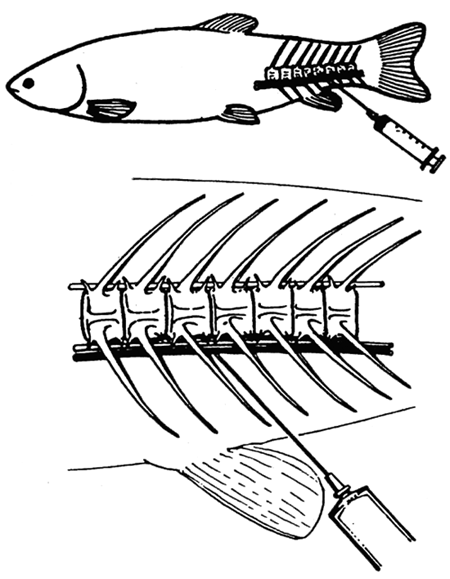
Fig. 22: Blood sampling in fish of more than 200 g weight by the method of punction of caudal blood vessels.
3/ Stabilization of blood
Aqueous solution of heparin sodium salt is the only product used for stabilization of the fish blood. One ml of this aqueous solution contains 5000 I.U. of heparin sodium salt. 0.01 ml (about one drop) of the aqueous solution of heparin suffices to stabilize 1 ml of fish blood: the substance is left to dry on the inner surface of the test tube or flask (bublet) and the blood is collected in the test tube or bublet afterwards. A slight overdosage of heparin does not produce changes in the blood cells of the fish.
Determination of the parameters of the red blood picture
1/ Erythrocyte count (Er, RBC)
The erythrocyte count in fish blood is determined in heparinized blood diluted by the Hayem solution at a ratio of 1:200. The solution has the following composition:
| mercury dichloride HgCl2 - sublimate | 2.5 g |
| sodium sulphate Na2SO4 | 25 g |
| sodium chloride NaCl | 5 g |
| distilled water | ad 1000 ml |
The flask (bublet) method after Bürker is used for the dilution of the blood. The blood is diluted in special glass bublets or in penicillin phials (volume 15 to 25 ml). First, a special pipette is used to put an accurate amount of 4975 μl of Hayem solution (filtered before use) into the bublet or phial; then 25 μl of heparinized blood is added, using a flushing micropipette. The micropipette is rinsed several times by repeatedly sucking the solution, the bublet is closed with a rubber stopper and its contents are stirred by circling motion for 2 to 3 min. A dropper or a Pasteur pipette is used to fill Bürker's counting cell with the diluted blood. The red blood cells are counted in 20 rectangles, regularly distributed over the whole lattice of the counting cell. The counting is usually done at a 200-fold magnification.
The resultant counted amount of erythrocytes is then reduced 100 times and the resultant value is the number of erythrocytes in T.1-1 (Tera = 1012). The erythrocyte count can still be determined by the traditional method after 24 hours of storage of heparinized blood at a temperature of up to 4 °C.
Colorimetric methods of determining the erythrocyte count in the blood of fish are introduced at present. The colorimetric method of determining the erythrocyte count after Pawinski, compared with the traditional method, is simpler, more rapid, less laborious, does not bear a subjective error, has a greater reproducibility, and is suitable for series determinations. The disadvantage is that its use in ichthyotoxicology is limited, especially in the study of the action of substances that increased the mean corpuscular volume.
The Pawinski solution having the following composition is used in the colorimethric method of determining the erythrocyte count in the blood of fish:
| anhydrous sodium sulphate Na2SO4 | 26.7 g or |
| sodium sulphate decahydrate Na2SO4. 10H20 | 67.9 g |
| sulphosalicylic acid | 2.0 g |
| distilled water | ad 1000 ml |
Blood is collected by means of a heparinized needle and dropped onto a watchglass from where 20 μl of blood is transferred by means of a rinsing micropipette into 10 ml of the Pawinski solution and is thoroughly stirred but not shaken. Sample extinction is measured against distilled water in a measuring cell (1 cm), using a filter at a wavelength of 600 nm. The erythrocyte count is read from the calibration curve drawn on the basis of parallel measurements of erythrocyte counts by the colorimetric method after Pawinski and by traditional method, using the Bürker counting cell. As far as possible, the measurement should be done within the whole variation range of erythrocytes in the blood of several tens of fish (30–40 fish usually suffice). An example of calibration curve is show in Fig. 23. When using the colorimetric method of determining the erythrocyte count, the blood sample should be diluted with the Pawinski solution straight on the spot of field examination, as soon as the blood is collected. The determination of extinction should be performed within 20 minutes to 4 hours after collecting the blood.
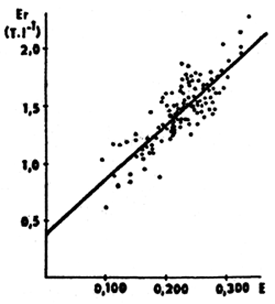
Fig. 23: Relationship between extinction (E) determined by the colorimetric technique after Pawinski and the erythrocyte count (Er) determined by means of the Burker counting cell
In the healthy carp, the erythrocyte count ranges from 1.1 to 1.8 T.1-1. In rainbow trout this range is from 0.80 to 1.50 T.1-1 of blood.
2/ Haemoglobin (Hb)
The cyanohaemiglobin method is used for determination of haemoglobin in the blood of fish. The principle of the method is that haemoglobin is released from the erythrocytes and transferred to cyanohaemiglobin by means of a transformation solution; the cyanohaemiglobin is then determined photometrically. Solution after van kampen and Zijlster can be used as the transformation solution. Its composition is as follows:
| potassium ferricyanide K3 [Fe(CN) 6] | 0.20 g |
| potassium cyanide KCN | 0.05 g |
| potassium dihydrophosphate KH2PO4 | 0.14 g |
| distilled water | ad 1000 ml |
The transformation solution is stored in a dark reagent bottle in a refrigerator and is replaced every 3–4 weeks. If left to freeze, the transformation solution is destroyed.
For the analysis of the blood to determine the haemoglobin, about 7 ml (or 5 ml) of the transformation solution is measured and poured into a test tube, a rinsing pipette is used to add 25 μl (or 20 μl) of fresh collected heparinized blood and the contents of the test tube are stirred immediately. The examination of heparinized blood for haemoglobin content should be performed within 24 hours after blood collection at the latest, if the blood is stored at a temperature up to 4 °C. The conversion of haemoglobin into cyanohaemiglobin is rapid: the data can be read from the photocolorimeter after 3 minutes. The cyanohaemiglobin colouring remains stable for 24 hours at the minimum. The sample extinction measurement itself is performed in a 1 cm cell at a wavelength of 540–546 nm against the transformation solution. The haemoglobin content is determined from the calibration curve. The calibration curve is drawn in the usual way, using the cyanohaemiglobin standard and the transformation solution.
Haemoglobin content in blood is expressed in g per litre. In healthy carp and trout its level ranges within the approximate limits of 60 and 100 g per litre.
3/ Haematocrit value (Hk, PCV)
The haematocrit value expresses the corpuscular volume in relation to the total volume of blood. In ichthyohaematology, heparinized capillaries 7.5 cm long are exclusively used for the determination of the haematocrit value. The freshly collected non-stabilized or heparinized blood (which may be left to stand at a temperature of up to 4 °C for 4 hours after collection at the maximum) is sucked into the capillaries to about ⅔ of their height and the clean end is sealed over a burner. Then the capillaries are put into the centrifuge (speed 14 000 r.p.m.) and are left to be centrifuged for 3 minutes. After centrifuging, the haematocrit percentage is directly read on the haematocrit meter which is a part of the haematocrit centrifuge set. The percent value obtained in this way is multiplied by coefficient 0.01 and the resultant value is the PCV in 1.1-1.
Owing to its simpleness and accuracy, determination of the haematocrit has become one of the basic examinations of the red part of the blood of fish. The physiological PCV values range from about 0.28 to 0.40 1.1-1 in carp and from 0.30 to 0.45 1.1-1 in rainbow trout.
4/ Basic erythrocyte data and their calculation
Mean corpuscular volume (MCV)
The value of the mean corpuscular volume can be calculated from the haematocrit value (PCV), expressed in 1.1-1, and from the erythrocyte count (Er), expressed in T.1-1. The following formula is used for this calculation:
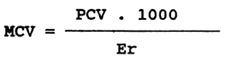
The value of the mean corpuscular volume, expressed in fentolitres (f1), ranges from 200 to 300 fl in healthy carp and from 350 to 400 fl in healthy rainbow trout.
Mean corpuscular haemoglobin (MCH)
Mean corpuscular haemoglobin expresses the average haemoglobin concentration in individual erythrocytes and is given in picogrammes - pg (10-12 g). It is calculated from the haemoglobin value in g.1-1 and from the erythrocyte count (Er) in T.1-1 according to the following formula:
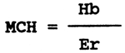
In carp, the optimum value of mean corpuscular haemoglobin ranges from 50 to 60 pg, in rainbow trout from 65 to 75 pg.
Mean corpuscular haemoglobin concentration (MCHC)
The mean corpuscular haemoglobin concentration expresses the concentration of haemoglobin in unit volume of erythrocytes. It is calculated from the haemoglobin value (Hb) in g.1-1 and from the haematocrit value (PCV), expressed in 1.1-1, according to the following formula:
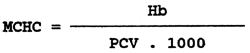
In healthy carp the value of the mean corpuscular haemoglobin concentration ranges from 0.20 to 0.26 1.1-1, in healthy rainbow trout from 0.17 to 0.20 1.1-1.
Determination of the parameters of the white blood picture
1/ Leucocyte count (Leuco)
The leucocyte count in fish is determined in heparinized blood, diluted with a solution after Procházka and Škrobák at a ratio of 1:200. The Procházka-Škrobák solution has the following composition:
| sodium chloride NaCl | 3.88 g |
| sodium sulphate Na2SO4 | 2.50 g |
| sodium monohydrophosphate dodecahydrate Na2HPO4.12H2O | 2.91 g |
| potassium dihydrophosphate KH2PO4 | 0.25 ml |
| formaldehyde 37% | 7.50 ml |
| brilliant cresil blue | 0.10 g |
| distilled water | ad 1000 ml |
Fresh (newly prepared) solution cannot be used for the determination itself: the solution must have been left to stand for about 2 weeks and should be filtered before use.
The flask method after Bürker is used for diluting the blood. Special glass flasks (bublets) or penicillin phials (volume about 15 to 25 ml) are used for the dilution: first, 4975 μl of the Procházka-Škrobák solution is put in the flasks or phial by means of a special pipette and then 25 μl of heparinized blood is added by means of a rinsing micropipette. The solution is then sucked repeatedly several times to rinse the micropipette, the flask is closed by means of a rubber stopper and its contents are stirred by a cycling motion for 2–3 minutes. A dropper or a Pasteur pipette is then used to fill Bürker's counting cells with the diluted blood. The leucocytes are counted in 100 large squares. The counting is usually done at a 200-fold magnification. The total number of leucocytes, counted in the 100 large squares, is multiplied by 0.5 to give the leucocyte count, expressed in G.1-1 (G-giga = 109). The leucocyte count can still be determined after 24 hours of storage of heparinized blood at a temperature of up to 4 °C.
The range of variation of the physiological values is very wide: in carp from 10 to 80 G.1-1 and in rainbow trout from 10 to 60 G.1-1.
2/ The leucocrit value (Lc, BC)
The leucocrit value expresses the leucocyte volume in relation to the total volume of blood. The leucocrit value is determined in heparinized microcapillaries simultaneously with the determination of the haematocrit value. The thickness of the leucocyte layer is measured under microscope using an eyepiece micrometer with a 60-fold magnification. Like the haematocrit value, the leucocrit value must also be determined within 4 hours after collection and storing heparinized blood at a temperature of up to 4 °C.
The technique of leucocrit value determination has until now been only developed for carp. The physiological values of leucocrit in carp range from 0.002 to 0.01 1.1-1.
3/ Differential leucocyte count (leucogramme)
Leucocytes include agranulocytes and granulocytes. The main distinguishing mark is the absence or presence of the differently coloured granules in the cytoplasm of these cells. Agranulocytes comprise lymphocytes and monocytes whose cytoplasm contains no granules, except sporadic azure grains which may occur in part of these leucocytes. The group of granulocytes comprises leucocytes whose cytoplasm usually contains a large amount of fine or coarser granules which differ from one another in staining capacity, i.e. ability to absorb either basic or acid colouring agents, or both. Depending on staining capacity, the granulocytes may be basophile, eosinophile or neutrophile. Besides the cytoplasm, the nucleus is another feature by which these cells are differentiated. The basic distinguishing traits are the shape of the nucleus, its size and internal structure. Large and compact nuclei are typical of agranulocytes. The nuclei of granulocytes are generally smaller, elongate in shape, and often divided into parts, or segments, connected by a thin strip of nuclear chromatin. As to the internal structure and density of the nucleus, lymphocytes rank first: they contain a dense and fine network of nuclear matter, the nuclei are compact and dense, giving a high degree of blue-mauve basic shade. On the other hand, the nuclei of granulocytes usually have a weaker basic tint.
The size of the lymphocytes ranges between 7 and 9 μ. Monocytes in size of 15 to 18 μ or even more in exceptional cases are among the largest cellular elements of the blood of fish. The size of the neutrophile granulocytes ranges between 5 and 10 μ. Eosinophile granulocytes are cells whose sizes range from 8 to 12 μ. Basophile granulocytes have a size about 10 μ.
The technique of determining the leucogramme of the blood of fish is as follows: the main point is to prepare the blood smear from the native blood immediately after sampling. Method of preparing the smears is pictured in Fig. 24.
The ground cover slip of the Bürker counting cell may be used as the smearing slip. The slides for the blood smears should have been washed with soap and water, rinsed and left in a chromium-sulphur bath. Pliers are used to remove the slides from the bath; then the slides are rinsed under flowing water and put into an alcohol bath where they are left for 12 hours at the minimum. Then they are taken from the bath and dried with a clean washed cloth. These slides are labelled as “fit for haematological use”.
The produced blood smears are left to dry. The fish blood smears should be stained as soon as possible: within four hours at the maximum from making the smears. For panoptic staining of the blood smears for the purpose of determining the leucogramme, the Pappenhaim staining method is used. The technique is as follows: cover the smear with about 30 drops of the May-Grünwald stain. Leave the stain to act for 3 minutes (in this stage the cells are only fixed with absolute alcohol of the staining mixture). After 3 minutes apply onto the May-Grünwald stain about 25 drops of neutral distilled water (its pH must be neither higher nor lower than 7) which, producing whirls, mixes with the alcohol of the mixture. In this way an aqueous solution is produced, having its own staining capacity. This stage of staining lasts two minutes. Then the staining solution is poured off the smears which, still wet, are then covered by a prepared solution of the Giemsa-Romanowski staining agent at a dilution ratio of 1:40 (1 part of stain, 40 parts neutral distilled water). It is mainly the cell nuclei that are stained during this stage, lasting 20 to 30 minutes. When 20 minutes elapse, the staining solution is removed from one (any) smear which is rinsed under flowing water and the staining effect is inspected at a small magnification under a microscope while the smear is still wet. If the cytoplasm of the cells is pink and the nuclei are blue, the staining is finished. Now the solutions are poured off all the remaining smears which are then rinsed under a stream of water and left to air-dry in vertical or slanting position. Microscopic analysis is done in oil immersion under a strong light, preferably at a 1000- to 1500-fold magnification. The slide with the smear is driven to move along a meander-like path under the microscope (Fig. 24). The leucocytes found during this process are classified and are recorded in a suitable table or fed to a digital computer. When the 200th cell is entered, the percentual proportions of the different kinds of leucocytes are determined. If accurate data on the total number of leucocytes are available, the leucogramme data may help also to provide valuable information on the absolute numbers of the different kinds of leucocytes.
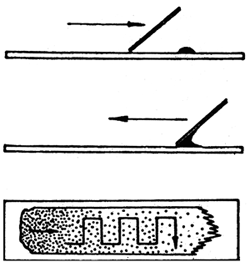
Fig. 24: Method of preparing the blood smears. Direction of smearing and examination.
The average leucogramme data (%) on carp and rainbow trout are shown here as examples:
| common carp | rainbow trout | |
| total lymphocytes | 92 | 93 |
| monocytes | 3 | 3 |
| neutrophile granulocytes | 4 | 4 |
| eosinophile granulocytes | 1 | 0 |
| basophile granulocytes | 0 | 0 |
Determining the values of selected biochemical characteristics in blood plasm
The collected heparinized blood must be centrifuged in cold conditions as soon as possible. If a cooled centrifuge is not available, a centrifuge with replaceable rotor can be used instead, the rotor having been cooled in a refrigerator before use. Owing to a lack of stability of the values of some parameters (ALT activity, glucose concentration) it is recommended to perform the determination as soon as possible after centrifuging the non-clotting blood (10 min, 100 G), or to store it at 4 °C or in frozen condition in microtubes with a seal.
Table 11. International system of SI units in ichthyohaematology on the example of normal values in the blood and blood plasm (pl) of carp
| Haematological parameter | variation range | SI units |
| Aminotransferase (AST)-pl. | 0.20–1.50 | μcat.1-1 |
| Aminotransferase (ALT)-pl. | 0.05–0.32 | μcat.1-1 |
| Ammonia -pl. | 200–800 | μmol.1-1 |
| Total protein (TP)-pl. | 20–40 | g.1-1 |
| Erythrocytes (Er,RBC) | 1.1–1.8 | T.1-1 |
| Glucose - pl. | 3–10 | mmol.1-1 |
| Glutamate dehydrogenase (CDH) - pl. | 0.35–0.75 | μcat. 1-1 |
| Haematocrit value (Hk, PCV) | 0.28–0.40 | l.1-1 |
| Haemoglobin (Hb) | 60–100 | g.1-1 |
| Mean corpuscular haemoglobin (MCH) | 50–60 | pg |
| Cholesterol - pl. | 1.5–12 | mmol.1-1 |
| Lactic acid (lactate) - pl. | 0.7–2.2 | mmol.1-1. |
| Lactate dehydrogenase, (LDH) - pl. | 3.5–9.5 | μcat.1-1 |
| Leucocytes (Leuco) | 10–80 | G.1-1 |
| Leucogramme: | ||
| - lymphocytes | 76–97.5 | % |
| - monocytes | 3–5 | % |
| - neutrophile granulocytes | 2–10 | % |
| - eosinophile granulocytes | 0–1 | % |
| - basophile granulocytes | 0–0.5 | % |
| Leucocrit value (LK, BC) | 0.002–0.01 | 1.1-1 |
| Total lipids (TL) - pl. | 2–10 | g.1-1 |
| Urea -pl. | 1–3 | mmol.1-1 |
| Mean corpuscular volume (MCV) | 200–300 | fl |
| Mean corpuscular haemoglobin concentration (MCHC) | 0.20–0.26 | 1.1-1 |
| Triacylglycerols - pl. | 1–4 | mmol1.1-1 |
Values of total protein (TP), total lipids (TL), glucosis, triacylglycerol, cholesterol, urea, ammonia, lactic acid, methaemoglobine, enzymes and other parameters are determined in blood plasm of fish. Analytic kits from producers Lachema Brno, Boehringen Mannheim, Hyland and others can be used for determinations. Orientation physiological ranges of these parameters are presented in table 11.
Recommended literature
Golovina N.A., Trombickij I.D. (1989): Haematology of pond fishes. Kišiněv, Štiinca, pp. 158. (in Russian)
Ivanova N.T. (1983): Atlas of fish blood cells. Moskva, izd. Legkaja i piščevaja promyšlennost, pp. 75. (in Russian)
Pravda D. (ed.) (1986): 1st ichthyohaematological conference (Litomyšl, 1985), Sborník přednášek a dokumentu. Brno, pp. 165. (in Czech)
Pravda D. (ed.) (1989): 2nd ichthyohaematological conference (Litomyšl, 1989). Sborník přednášek, Praha, pp. 350. (in Czech)
Svobodová Z., Pravda D., Paláčková J. (1991): Unified methods of haematological examination of fish. Research Institute of Fish Culture and Hydrobiology, Vodňany, pp. 31.
