by
Edgardo D. Gomez1
SUMMARY
1. INTRODUCTION
This introductory lecture is meant to review in broad terms the general characteristics of the marine environment. A basic overview of marine ecology is provided by E. P. Odum (1971) in his book Fundamentals Of Ecology.
2. FEATURES OF THE SEA
Features of the sea of major ecological interest are the following:
2.1 Vastness of the oceans - 70 percent of the earth's surface
2.2 Depth - life is found from the surface to the trenches
2.3 Continuity - no complete land barrier
2.4 Circulation is continuous and movement is effected by waves, titles and currents
2.5 Salinity - salts make up 3.5 percent of seawater
2.6 Concentration of nutrients is generally low except in shallow areas and upwelling zones.
3. VARIOUS ZONES OF THE SEA
The sea may be divided into various zones based on the following criteria:
3.1 Light penetration: euphotic; aphotic
3.2 Depth of bottom: continental shelf; continental slope; abyssal plains; mid-ocean ridges; trenches, canyons and ridges
3.3 Distance from shore: neritic; oceanic or pelagic
4. FOCUS OF STUDY
In this training course, special attention will be focused on the shallow littoral fringe: intertidal and shallow subtidal. Seaweeds naturally occur abundantly in this zone. Harvesting of natural stocks occurs in the open shallow subtidal zone. Ponds are constructed on the intertidal and shallow subtidal. Depending on the tidal characteristics of each location, the ponds may be closer to the natural shore or farther out.
An understanding of the physical, chemical and biological characteristics of the area in question is useful in planning pond development and seaweed culture. Ideally, a total ecosystem study should be undertaken in planning seaweed culture activities.
(Detailed discussions of the above topics were rendered during the training course).
by
Gavino C. Trono, Jr. 1
1. INTRODUCTION
The environmental conditions regulate the kind, nature, abundance and productivity of seaweed communities.
The stature of the community is reflective of the environmental conditions in the area.
Different kinds of species are found in certain habitats because environmental conditions are favourable to their development.
2. ENVIRONMENTAL FACTORS
The ambient ecological condition in a certain habitat is the result of the combined and synergistic effects of the various ecological factors. Some of these factors may exert modifying effects on the others. The understanding of the influence of these parameters on the nature, biology and distribution of seaweeds is important in the evaluation and assessment of sites for mariculture purposes,
2.1 Salinity
Salinity is defined as “the total amount of solid materials in grams contained in one kilogram of seawater when all the carbonate has been converted to oxide, bromine and iodine replaced by chlorine and all the organic matter completely oxidized”,
Most seaweed species cannot withstand exposure to a wide variation in salinity regimes while others can. Those which cannot tolerate wide range of salinity regimes are known as stenohaline species while those which can can are called euryhaline species. The effects of salinity on the structure of seaweed communities may be best illustrated by comparing the variety of species found in habitats which are influenced by freshwater (brackish) with those far from freshwater sources. Areas influenced by freshwater are generally characterized by low -species diversity compared to reef areas not influenced by low salinity. The low diversity may be mainly due to the fact that only few species can thrive in habitats with highly fluctuating salinity regimes. For instance, certain species like Gracilaria verrucosa thrives very well in brackishwater areas while other species will die in such habitats.
2.2 Light and temperature
All photosynthetic plants require light as source of energy for the synthesis of organic products necessary for their normal growth and development. The different groups of seaweeds possess pigments of various types which enable them to adapt to different light conditions in the sea.
The quality and intensity of light change as it penetrates the water column. The change in light quality and intensity affects the distribution of the various species. The influence of light on seaweed distribution is best illustrated by their vertical zonation. In general, those with pigments adapted for low intensities may be found in deeper areas while those adapted to full or bright light conditions are found in shallow areas.
Heating of the water is a secondary effect of light which also affect the local distribution of seaweed species. Such effects, however, are limited to shallow intertidal areas or tidepools which become isolated and are not influenced by free water circulation. In such habitats, the temperature may become very high and can cause the exclusion of many species from the area. Such pronounced increase in water temperature takes place especially when low. tides occur during the day. There is no significant change in water temperature in deeper areas where the water is constantly mixed by currents or wave action.
2.3 Substratum
Substratum provides mechanical support or attachment for the seaweeds. Seaweeds have different types of attachment organs adapted to various types of substrata. For instance, those species found in sandy-muddy habitats are generally characterized by fine and penetrating rhizoidal holdfasts while those in solid, hard or rocky substrates are characterized by strongly developed, branching or discoidal holdfast. Thus, the different types of substrate influence the composition and local distribution of seaweed species. The awareness of the role of substrates on the local distribution of seaweeds is, thus, very important in inventory and stock assessment survey work.
2.4 Water movement
Water movement is an overall primary factor which control or influence the nature or status of seaweed communities as well as modify or moderate the extreme effects of other ecological factors. Water movement caused by waves and currents is important in aeration of the water, nutrient transport and mixing which prevents the rise in water temperature as well as light penetration. It also influences the amount of harvestible seaweed stocks. Waves are known to mechanically remove significant amounts of seaweed stocks. This effect is best illustrated by the tremendous amounts of drifted seaweed materials which accumulate along the beaches after storms. Wave-exposed areas are not good sites for the mariculture of seaweeds because of the destructive effects of waves on the farm. On the other hand, areas influenced by moderate currents are preferred sites for farming of certain species such as Eucheuma because currents are less destructive. Productive Eucheuma farms in the Philippines are characterized by moderate to strong currents. Loss of plants due to removal by currents can be significantly controlled by the construction of catchments nets at the leeward side of the farm.
2.5 Water depth
The water depth is another important factor which influences the local abundance and distribution of seaweeds. The upper limit of the vertical distribution of seaweeds is closely related to the upper tidal levels while the lower limit is limited by the amount of light which penetrates the water column. In general, most seaweed communities are well developed starting from approximately plus or minus the 0 datum level to a few meters below this depth. The upper limit of vertical distribution of seaweed is influenced by the amount of exposure to air and sunlight during low tides and the inherent capacities of the species to resist dessication and the complications brought about by changes in salinity and water temperature during such exposure. The lower limit is generally related to the light conditions. Few species can thrive well in very low light intensities in deeper areas.
The understanding of the role of water depth in the vertical distribution and growth of seaweeds has a very important bearing in the selection of good sites for seaweed farms.
2.6 Biotic factors
One of the ever present factors which controls the marine vegetation are the associated animal life. Seaweeds and seagrasses are constantly being grazed upon by a host of animal grazers. These animals significantly control the amount of harvestable organic matter (biomass) in reef areas. Studies have shown that when grazing is controlled by the physical destruction or removal of grazers, luxuriant growth of seaweeds would follow.
Fungal and bacterial diseases are recognized as important biotic factors which influence, the productivity of seaweed communities. These two factors are important considerations in the farming of seaweeds. Seaweed crops may be completely lost due to the destructive effects of these biotic factors. The presence of “weeds” is also one of the major problems in farms. These unwanted seaweed species compete with the crop plants for nutrients, space and light.
2.7 Other factors
Dissolved gases such as oxygen and carbon dioxide seldom become limiting factors to the growth and development of seaweed communities. These gases are abundant in sea where there is continuous mixing of water due to the action of waves and currents. Oxygen comes from the two major sources, i.e. from the atmosphere and as by-product of photosynthesis. Aeration of the water column is facilitated by waves and currents. Carbon dioxide which is a principal raw material in the production of organic substances through photosynthesis is present in abundance in the sea as dissolve gas or in the form of carbonate compounds. Another factor which seldom becomes limiting in the sea is pH. The buffered nature of seawater does not allow extreme fluctuation in pH values except in special types of habitats such as tidepools and isolated shallow lagoons especially during low tide regimes.
by
Edna T. Ganzon-Fortes1
1. SEAWEEDS IN GENERAL
There are four major divisions of marine algae, namely, the Cyanophyta or blue-green algae, the Chlorophyta or green algae, the Phaeophyta or brown algae, and the Rhodophyta or red algae. The macroscopic and benthic forms of marine algae constitute what we commonly term as “seaweeds”. Because of the usual inconspicuous nature of the blue-green algae in the marine environment, the abundant green, brown and red algae traditionally comprise the seaweed group. They vary in size, form and colour and they are found in various kinds of habitat along the shore, in the supralitoral, littoral and sublittoral areas, attached to various kinds of substrate such as sand, mud, rocks, shells, pieces of coral, boulders, wood, even to sea grasses or to other seaweed species.
2. CHARACTERISTICS OF SEAWEEDS: STRUCTURE AND REPRODUCTION
2.1 Seaweeds versus higher plants
Seaweeds may be similar in form with the higher vascular plants, but the structure and function of their body parts significantly differ from the higher plants. They do not have true roots, stems or leaves. The body of a seaweed is called a thallus (plural: thalli) which consists of the following basic parts:
The holdfast may resemble the root of the higher plants but, both its structure and function are markedly different. The holdfast's primary function is for attachment. It does not function for absorption. It may be discoid, rhizoidal, bulbous, or branched, depending on the substratum to which the seaweed is adapted to.
The stipe resembles the stem of the higher plants. The stipe, however, mainly functions for support of the blade, for photosynthesis and for absorption of nutrients from the surrounding water. However, the stipe of some “kelps” (the giant brown seaweeds found in temperate waters) have medullary cells which have similar structure and conductive function with the phloem of the higher vascular plants. Yet, the absence of true vascular tissues in the kelps (i.e. both xylem and phloem) sets this group apart from the higher plants.
The blade of the seaweeds may resemble the leaves of the higher plants. However, it is very variable in form (it may be smooth, ruffled, perforated, segmented, dentate, undulate, etc.) and functions not only for photosynthesis and absorption of nutrients from the surrounding waters, but also for reproduction. It contains or produces the reproductive organs.
The most significant difference of the seaweeds from the higher plants other than those mentioned above, is that, their sex organs and sporangia are usually one-celled, or if multicellular, their gametes and spores are not enclosed within a wall formed by a layer of sterile or non reproductive cells.
2.2 Characteristics of the four major groups of marine benthic algae
2.2.1 Cyanophyta (blue-green algae)
The majority of the blue-green algae are found in freshwater, terrestrial and aerial habitats. Few representatives inhabit brackish and marine waters. They are characterized by the low state of cellular differentiation and thallus organization. The members are either coccoid or filamentous and they form colonies of various sizes. Their colour varies from dark bluish-greenish, brownish, or pinkish-reddish depending on the proportion of the pigments present in their cells such as the chlorophylls (green) and phycobilins [(c-phycoerythrin (red) and c-phycocyanin (blue)]. Unlike the three other groups, their pigments are not contained in plastids. Reproduction in this group is purely asexual by fission, fragmentation, or spore formation.
In the marine environment, few members of the filamentous group may be encountered mixed or associated with the other benthic algal species. In certain occasions, some course filamentous members may form thick tufts on the substrates. This is most common in seaweed farms where they form a significant component of the weeds, thus, becoming a nuisance and a problem. The blue-green species, Lyngbya majuscula, is the most common weed which forms thick tufts on the nets and posts of Eucheuma farms or on the Eucheuma thallus itself. Two other species, Symploca hydnoides and Schizothrix calcicola, are also minor components of some reef areas in the Philippines.
2.2.2 Chlorophyta (green algae)
The green algae are mostly found in freshwater habitats although few members are truly marine forms. They are so named because of their grass-green colour due to the predominance of chlorophylls a and b over the carotenes and xanthophylls. These pigments are contained in special cell structures known as chromatophores. The cell wall of the green algae is composed of an outer layer of pectin and an inner layer of cellulose. Starch is the photosynthetic product of this group.
The members range from unicellular to multicellular, microscopic to macroscopic forms. The thalli of the macro-benthic forms of green algae vary from free filaments to definitely shaped forms which may consist of a blade, stipe and holdfast. The photosynthetic portions of the thalli may not be or may be moderately too highly calcified, appearing in a variety of forms - as fan-shaped segments, feather-like or strap-shaped branches with teeth or pinnules, clavate or globose branchlets and others.
Reproduction among the green algae is sexual and asexual by the formation of flagellated spores and, less frequently, by the production of non-flagellated spores. Vegetative multiplication through fragmentation is also common especially among the filamentous species.
2.2.3 Phaeophyta (brown algae)
The brown algae are almost exclusively marine forms. The pigments present in their cells are chlorophylls a and c, carotenes and the xanthophyl1, fuxoxanthin, which is responsible for their brown colour. Their cell wall is composed of an outer layer of algin and an inner layer of cellulose. Laminarin and mannitol are the food reserves of this group.
The brown seaweeds have forms varying from simple, freely branched filaments to highly differentiated forms consisting of distinct blades, stipes, and holdfasts as exhibited by the giant kelps (i.e. Fucus, Laminaria and Macrocystis) found in temperate waters.
Several species of the brown algae reproduce vegetatively by fragmentation of the thallus. This may take place at the juvenile or adult stage. Vegetative propagation may also be due to the formation and abscission of special reproductive branches known as propagulla. All members of this group except members of Fucales, produce biflagellated neutral spores found within one-celled or many-celled reproductive organs. Sexual reproduction through the union of flagellated male and female gametes or union of flagellated male and large non-flagellated female gametes also occur commonly among the browns. Alternation of the gametophytic and sporophytic generations occur in this group except in the members of Fucales.
2.2.4 Rhodophyta (red algae)
The red algae, except for a few species, are exclusively marine forms. The predominance of the red pigment, r-phycoerythrin, over the others, give the plants their distinctive red colour. Similar to the green algae, their cell wall is also composed of an outer layer of pectin and an inner layer of cellulose. Their food reserve is Floridean starch.
They vary in size and form - as small epiphytes, as thin crust on the substratum, or as large, fleshy, branched, or blade-like thalli.
The red algae seldom reproduce vegetatively by fragmentation of the thallus. All of the members produce one or more kinds of non-flagellated spores which are either sexual or asexual in nature. Sexual reprpduction in Rhodophyta is unlike that of any other algae and is very complicated. It involves several structures after the union of the male-female gametes. Some members of this group exhibit a biphasic alternation of generation in which a sexual generation (the gametophyte) alternates with an asexual generation (the tetrasporophyte). Others are triphasic with three generations or somatic phases (gametophyte, carposporophyte, tetrasporophyte) successively following one another.
3. ECONOMIC IMPORTANCE OF SEAWEEDS
Seaweeds have a variety of uses in man's everyday life. It is used as food, fodder, fertilizer, and medicine. As fodder, it is food for goats, cows, sheeps, horses, poultry and hogs. As medicine, it is used for the treatment of goiter and other glandular troubles. It is also used as vermifuge, or for the treatment of diarrhea and other stomach or urinary disorders.
Seaweeds are also sources of important raw materials such as agar, carrageenan, and algin which have various uses in industries. These are used as thickening, suspending, stabilizing, emulsifying, gel-forming, and film-forming colloids. Algin provides ice cream its smooth texture by preventing formation of ice crystals. It is used as a suspending agent in auto polishes, in paints, in Pharmaceuticals, in drugs and antibiotics. As a stabilizing agent, it serves in the processing of rubber latex and in the printing of textiles. It is also widely used as an emulsifier in water-base paints, french dressings and cosmetics. Agar, on the other hand, is used in bacteriology for the formation of the medium for the culture of bacteria. As a food adjunct, it is used as gelatin, anti-drying agent in breads and pastry, in improving the slicing quality of cheese, in the manufacture of frozen dairy products, etc. In industry, it is used for the waterproofing of paper and cloth, in photographic films, shoe polish, dental impression molds, shaving soaps, and hand lotions. In tanning industry, agar imparts stiffness and gloss to finished leather. Carrageenan resembles agar but it has high ash content and requires higher concentration to form gels. It is used in the making of surgical jellies, salves and ointments. Also, it acts as a stabilizing agent in ice cream, sherbets, and other frozen dairy products.
In the Philippines, quite a number of seaweeds have economic potentials as food, medicine, fertilizer and as sources of industrially important colloids. A list of these seaweeds is shown in Table 1.
4. REFERENCES
Dawson, E.Y. 1966 Marine botany, an introduction. Holt, Rinehart and Winston, Inc. New York, Chicago, San Francisco, Toronto, London. 371pp.
Smith, G.M. 1955 Cyptogamic Botany, Volume I - Algae and fungi. McGraw-Hill Book Company, Inc. New York, Toronto, London. 546pp.
Table 1. List of economically important seaweed species in the Philippines*
| Name of species | Economic importance | |
| CHLOROPHYTA | ||
| Cladophora spp. | Food for milkfish | |
| Chaetomorpha aerea | Food for milkfish | |
| C. crassa | Human food | |
| Caulerpa lentillifera | Human food | |
| C. racemosa | Human food | |
| C.peltata | Human food | |
| C.sertularioides | Human food | |
| C.taxifolia | Human food | |
| Codium edule | Human food | |
| C. intricatum | Human food | |
| C. papiilatum | Human food | |
| C.tenue | Human food | |
| Halimeda tuna | Fodder | |
| Ulva fasciata | Human food | |
| U. lactuca | Human food; source of Vitamin E; | |
| U. prertusa | Human food; medicine | |
| Enteromorpha compressa | Human food | |
| E. intestinalis | Human food; source of Vitamin E; food for milk fish | |
| E. lingulata | Human food | |
| E. plumosa | Human food | |
| E. prolifera | Human food | |
| Monostroma nitidum | Human food | |
| PHAEOPHYTA | ||
| Padina commersonii | Human food | |
| P. tetrastomatica | Human food | |
| Hydroclathrus clathratus | Human food; nutritive value; iodine, mannitol; fodder; fertilizer; source of algin | |
| Sargassum cristaefolium | Nutritive value: protein; source of algin; fertilizer; source of methane | |
| (S. duplicatum | -do- | |
| S. enerve | -do- | |
| S. granuliferum | -do- | |
| S. hemiphyllum | -do- | |
| S. ilicifoliumum | -do- | |
| S. siliquos | -do- | |
| S. polycystum | -do- | |
| Turbinaria conoides | Human food; source of algin; fertilizer | |
| T. ornata | -do- | |
| RHODOPHYTA | ||
| Porphyra suborticulata | Human food; aphrodisiac; source of agar | |
| P. crispata | -do- | |
| Liagora farinosa | Human food | |
| Asparagopsis taxiformis | Human food; fodder | |
| Scinaia sp. | Human food | |
| Gelidium crinale | Source of agar | |
| G. puchellum | Source of agar | |
| G. pusillum | Human food | |
| G. rigens | Source of agar | |
| Gelidiella acerosa | Human food; source of agac | |
| Hypnea cervicornis | Source of carrageenan | |
| H. musciformis | Human food; nutritive value: fat, protein, iodine; source of growth hormone, gibberellin; medicine (as vermifuge); source of carrageenan | |
| H. valentiae | Human food; nutritive value: protein; source of carrageenan | |
| H. pannosa | -do- | |
| Catenella impudica | Human food | |
| Eucheuma arrioldii | Human food; source of carrageenan | |
| E. cottonii | Human food; source of carrageenan | |
| E. procrusteanum | -do- | |
| E. serra | -do- | |
| E. spinosum | -do- | |
| E. striatum | -do- | |
| Gracilaria arcuata | Source of agar | |
| G. compressa | Source of agar | |
| G. coronopifolia | Human food; nutritive value: fats, protein, Vitamin C; source of agar | |
| G. crassa | Human food; source of agar | |
| G. eucheumoides | Human food; source of agar | |
| G. lacinulata | Source of agar | |
| G. lichenoides | Human food; nutritive value: protein, fats, Vitamin C; source of agar; medicine | |
| G. salicornia | Human food; source of agar | |
| G. verrucosa | Human food; source of agar | |
| Gracilaria sp. | Human food; source of agar | |
| Acanthophora spicifera | Human food; source of carrageenan | |
| A. muscoides | Human food; source of carrageenan | |
| Bostrychia radicans | Human food | |
| Digenia simplex | Source of agar; medicine (anthelmintic) | |
| Laurencia cartilaginea | Human food | |
| L. obtusa | Human food; nutritive value: carbohydrates, Vitamins-folic and folinic acids; medicine (antibiotic and antifungal) | |
| L. papillosa | Human food; nutritive value: carbohydrates; Vitamins-folic and folinic acids; medicine (antibiotic and antifungal); source of agar and carrageenan | |
| L. okamurai | Human food; nutritive value: Vitamins-folic and folinic acids; source of chemical products; possible source of agar and carrageenan | |
| Halymenia durvillaei | Human food | |
| Grateloupia filicina | Human food | |
REFERENCES
Abbot, I.A. 1974 Limu, an ethnobotanical study of some edible Hawaiian seaweeds. Pacific Tropical Botanical Garden. 21pp.
Bersamin, S.V., R.B. Banania and R. Rustia. 1967 Protein from seaweeds, for animal feed substitutes. Phil. J. Sci. 96(2): 159–175.
Boney, A.D. 1965 Aspects of the biology of the seaweeds of economic importance. Pages 105–253 in F.S. Russel (ed.). Advances in Marine Biology. Academic Press, London and New York.
Cajipe, A., F. Laserna, R. Veroy and A. Luistro. 1980 On the infrared spectrum of polysaccharide obtained by alkaline extraction of the red algae, Acanthophora spicifera (Vahl) Boergesen. Bot, Mar. 23: 69–70.
Chapman, V.J. 1962 The algae. London MacMillan & Co. Ltd., London, New York, Toronto. 472pp.
Chase, F.M. 1942 Useful algae. Smithsonian Report for 1941. Smithsonian Institution Washington, D.C. pp. 402–452, 9 pls.
Dawson, E.Y. 1966 Marine botany, an introduction. Holt, Rinehart and Winston, Inc. U.S.A. 301pp.
Galutira, E.C. and G.T. Velasquez. 1963 Taxonomy, distribution and seasonal occurrence of edible marine algae in Ilocos Norte, Philippines. Phil. J. Sci. 92(4): 483–522, 9 pls.
de Leon, A.I., N. Eufemia and M. Pineda. Chemical composition of some Philippine algae. Phil. J. Sci. 92: 77–87.
Hoyle, M.D. 1975 The literature pertinent to the red algal genus Gracilaria in Hawaii. Marine Agronomy Program of the University of Hawaii, Department of Botany, Technical Report No. 3. 340pp.
Isaac, W.E. and C.J. Moltens. 1953 Seaweed resources of South Africa. Proc. Intl. Seaweed Symp. 1: 101–102.
Jensen, A. 1976 Tocopherol determination in seaweeds. Pages 281–286 in Proc. Int'l. Seaweed Symposium, 1976.
Kanazawa, A. and D. Kakimoto. 1958 Studies on the vitamins of seaweeds - I. Folic and Folinic Acid. Bull. Jap. Soc. Fish. 24 (6–7): 573–577.
Michanek, G. 1975 Seaweed resources of the ocean. FAO Fisheries Technical Paper No. 138. 127pp.
Moreland, P.S. 1973 Edible seaweeds of Northern Luzon Philippines: market prices, local taste preference, seaweed recipes and other local uses. (unpublished)
Mshigeni, K.E. 1974 An extended review of the literature on Hypnea, a red algal genus. Marine Agronomy Program, University of Hawaii, Department of Botany, Technical Report No. 2. 221pp.
Mshigeni, K.E. and E.V. Nzalalila. 1977 Contributions on the content and nature of the phycocolloid from Laurencia papillose (Forsak.) Grev. (Rhodophyta, Ceramiales). Bot. Mar. 30: 443–447.
Ramarao, K. and V. Krishnamurty. 1968 Study of the preparation and properties of the phycocolloid from Hypnea musciformis (Wulf.) Lamouroux from Veraval, Gujarat Coast. Bot. Mar. 11: 129–133.
Santelices, B. 1974 Gelidioid algae, a brief resume of the pertinent literature. Marine Agronomy Program, University of Hawaii, Department of Botany, Technical Report No. 1. 111p.
Schrumm, G.M. 1953 Canadian (Pacific Coast) Seaweed Resources. Proc. Int'. Seaweed Symp. 1: 107–108.
Sulit, J.E. and R.C. San Juan. 1955 Studies on the extraction of alginic acid from some species of Philippine Sargassum. Phill. J. Fish. 3: 47–53.
Suto, S. 1953 Seaweed production and phycological research in Japan. Proc. Int'l. Seaweed Symp. 1: 96–99.
Trono, G.C., Jr. 1973 Seaweeds: one of the important marine natural resources of the Philippines in the Gregorio T. Velasquez Lecture Series. Challenge to the biologists in the 70's: the escalation of food production. NRCP Publication. 28–4lpp.
Rodriguez, O. 1953 Seaweeds of industrial interest in the Canary Isles. Proc. Int'l. Seaweed Symp. 1: 75–81.
Velasquez, G.T. 1953 Seaweed resources of the Philippines. Proc. Int'l. Seaweed . Symp. 1: 100–101.
Velasquez, G.T. 1968 The edible seaweeds of the Philippines. The Philippine Biota. 2(3): . 118–123.
Velasquez, G.T. 1971 Studies and utilization of the Philippine marine algae. Proc. Int'l. Seaweed Symp. 7: 62–65.
Velasquez, G.T, G.C. Trono, Jr. and M.S. Doty. 1972 Algal species reported from the Philippines. Phil. J. Sci. 101(3–4): 115–169.
by
Miguel D. Fortes 1
1. INTRODUCTION
Much of our knowledge on seaweed research is based, to a very large extent, on samples. An administrator's choice of a farming technique is often determined by the “impressions” gathered from new encounters, he has had with the technique in the course of a few years. He would differ from one who devotes 20 years to “trial-and-error” methods, “feeling” the best possible method for optimum seaweed production. But in a real sense, the two differ in that the former bases his conclusions on a much smaller sample of experience. In science and in human affairs alike, often we lack the resources in order to take a complete study of the phenomena that might advance our knowledge. This is the primary reason why investigators resort to gathering samples.
Administrators accustomed to dealing with complete censuses as bases of policies are suspicious of samples and are reluctant to use them.
2. NON-SCIENTIFIC AND SCIENTIFIC SAMPLING
Non-scientific approaches to ecological studies may be in the form of:
Unfortunately, all these approaches are usually very biased, even though not intentionally so. If sufficient data from previous experience or originally gathered by someone else is available, it may be used as the basis for decision-making. But often, this is not so easy to secure, or if readily available, insufficient for the purpose. Thus, the need arises to collect primary or new data.
Values reported from “spot checks” may be accurate but one cannot be assured that the conclusions drawn from them are valid and reliable. It is risky when used as a basis for making programme-management decisions. With 100 percent surveys, their costs are prohibitive, the actual surveys very time-consuming, and they are often physically impossible to conduct.
Scientific sampling is “... the use of efficient and effective systematic methods of collecting, interpreting and presenting data on a quantitative manner to facilitate understanding.” With these methods, bias inherent in the non-scientific techniques can be appreciably eliminated and the probability of being correct (or incorrect) ascertained. The principal advantages of scientific sampling as compared with complete enumeration in ecological studies are as follows:
Subjective and personal bias in the overall methodology is minimized;
Inasmuch as the conclusions will be based on just a few samples, precise quantitative statements can be made regarding how these samples closely reflect the whole population from where they were drawn;
The probability of being correct or incorrect can be estimated.
Since scientific sampling can give smallest number of samples needed to reflect the characteristics of a population, the method becomes more economical, efficient and effective; and
Compared to 100 percent survey, scientific methods are the more accurate and practical because there are many different sources of error in any enumeration of large amount of data. Hence, the smaller (but the more carefully controlled) the sample, the less chances of making mistakes.
3. SOME GENERAL GUIDELINES IN CONDUCTING A SCIENTIFIC STUDY
3.1 Clarification of objectives
It is often the case that your head office just wants “to know something about” or to be kept informed on the status of key areas in the project. There really is no “problem” and the request for you to study an area usually comes in the form of ambiguous formulae, focused on some observed details that seem to bother management. Get your guidance clear on what you are to study and once the objectives have been definitely stated, the need for the study becomes clearer.
The questions to be answered include: Why does management want to study? How important does the head office consider the need for answers? How accurate do the results need to be? When does management want the results? What is the budget limitation of the study?
3.2 Planning and organizing the study
There are many factors to consider in planning and organizing the survey. However, these can be categorized under two general headings, namely, administrative and technical. A serious consideration of funds, staff, equipment, and administrative coordination is necessary before you go any further. Technically, once the problem is understood, formulate hypotheses to serve as possible explanations for the phenomenon to be encountered. This will lead to the right kinds of questions to be asked in order to resolve which of the hypotheses are correct.
Sometimes, questionnaires are important. The design and format of such materials are very significant in the accuracy in recording data. Master lists and a working schedule must be made available in order to complete the work in time for management's use. Also, an appropriate sample size must be determined as this will limit your time, money, and personnel.
3.3 Selecting a sample
In sampling, one must know exactly the natural entity to be sampled. For example, if we obtain a collection of seaweeds from within a 0.25 m2 plot, we must be aware that we have collected only a fraction of the entire vegetation. No single sampling technique can possibly give all the data required of a natural population or community. A given procedure can be best applicable only to certain species or group of species and not to others. This is the reason why the entity to be sampled must first be defined.
The population to be sampled (the sample population) should coincide with the population about which information is wanted (the target population). Conclusions drawn from the sample apply to the larger population.
3.4 Selecting a sampling procedure
There are several approved methods for drawing samples from a population, each of which has certain advantages depending upon the specific circumstances. If such item in the population is considered to have “equal importance” or where every point in that area is exactly like every other, you can perform either a SIMPLE or a SYSTEMATIC SAMPLING, the latter, useful especially if your population is arranged or lined up or along the environ-mental gradient. If on the other hand you know that the characteristics of the items in the population differ markedly, and it is possible to classify them you might want to select samples from each of these groupings in order to improve the validity of the survey. This more sophisticated approach is known as STRATIFIED SAMPLING. Finally, as plants may occur in clusters, it is possible to choose a number of clusters at random and measure the particular variable being studied on each of the units or individuals in the cluster. This method is called CLUSTER SAMPLING. Due probably to difficulties in field travel in some situations and/or in order to reduce travel time and cost, this technique may be the only practical means available to conduct the study.
The above sampling methods usually involve plots or quadrats of measured area. Hence, the results obtained using them are likely to be a function of the plot size. In plotless sampling, there is the advantage of not having to demarcate plots of a. certain size or shape (hence, less laborious). The most popular of the plotless techniques are the POINT-QUARTER (QUADRANT) and WANDERING-QUARTER methods.
3.5 Conducting the survey
Once the ecological entity has been defined and the sampling procedure chosen, one can do the actual sampling. How to obtain representative samples of the defined population is the next problem. Normally, statistical samples should be taken at random, in which case, you assume that each item in the population has an equal chance of being “picked” and that the occurrence of one individual in a sample in no way influences the inclusion of another. A sampling method is biased if it tends to underestimate a characteristic of the population or a community.
In a taxonomic sense, representative samples are those complete in parts (i.e. sample seaweeds to be collected should have fronds, stipes and holdfasts). Only such whole plants can facilitate correct and proper identification.
A single measurement is generally not enough to be used as basis of conclusions about an ecological characteristics. Replicates should be taken and from them, the mean of the statistical population can be estimated and the amount of error, determined.
In some instances, only portions of ecological samples (or subsamples) can be taken in the laboratory and studied. So long as the subsamples are taken randomly from the sample, they reflect the characteristics of the entire sample.
3.5.1 Subjective assessment of abundance
(a) Frequency ratings
The simplest and most rapid approach in describing a vegetation is to list the species present within a sample area and to attach to each, a subjective rating of its abundance. One common rating employed by ecologists makes use of the terms dominant, abundant, frequent, occasional and rare, qualified by the prefixes, “very”, and “locally” when necessary. For example, a clumped habit of the red seaweed, Gracilaria, scattered over an area, would be considered locally frequent.
The drawbacks of this method in describing a vegetation arise primarily from factors which unknowingly influence the judgement of the worker. Small species tend to be rated lower than bigger ones; those that form conspicuous clumps would be preferentially classed as abundant while those which are more evenly scattered in the area, less abundant. One species referred to as frequent by one field worker may be classed as occasional by another. Furthermore, the word “dominant” carries with it two meanings: in the physiognomic sense, it describes that species exerting the most influence, probably by virtue of its number and adaptability, on the other species in the community. For example, in terms of number of clumps, the smaller red seaweed Gelidiella may be the physiognomically dominant species in a sample area, but due to the “cleaning” or “blanketing” effect of the bigger brown seaweed, Sargassum, the latter, although fewer, may very well be the sociologically dominant species. It is therefore recommended that instead of the term dominant, the frequency symbol, very abundant should be used. To all intents and purposes, the two are synonymous.
(b) The Braun-Blanquet's system
A better defined approach in describing vegetation has been proposed by Braun-Blanquet (1927). Two scales are used, one, combining the number and cover of a species, and another, giving a measure of the groupings.
| + | = | sparsely or very sparsely present; cover very small |
| 1 | = | plentiful but of small cover value |
| 2 | = | very numerous, or covering at least 1/20 of the area |
| 3 | = | any number of individuals covering 1/4 to 1/2 of the area |
| 4 | = | any number of individuals covering 1/2 to 3/4 of the area |
| 5 | = | covering more than 3/4 of the area Society |
| Society 1 | = | growing singly, isolated individuals |
| Society 2 | = | grouped or tufted |
| Society 3 | = | in small patches or cushions |
| Society 4 | = | in small colonies, in extensive patches or forming carpets |
| Society 5 | = | in pure populations |
This system of rating, however, also suffers from unconscious errors due largely to bias of different recorders.
(c) The Domin scale
One of the several modifications of the Braun-Blanquet scale is the Domin scale which makes use of an increased number of divisions. This method assures workers of a high degree of accuracy, the reason why it is widely used. One other advantage is that it can be converted directly into the Braun-Blanquet scale when necessary.
| Domin Scale | Braun-Blanquet Scale | |
| Cover about 100% | 10 | |
| 5 | ||
| Cover 75% | 9 | |
| Cover 50–75% | 8 | 4 |
| Cover 33–50% | 7 | |
| 3 | ||
| Cover 25–33% | 6 | |
| Abundant, cover about 20% | 5 | 2 |
| Abundant, cover about 5% | 4 | |
| Scattered, cover very small | 3 | |
| 1 | ||
| Very scattered, cover small | 2 | |
| Scarce, cover small | 1 | |
| Isolated, cover-small | X | X |
3.5.2 Quantitative assessment of abundance and production
Obviously, a large amount of error is inherent in using subjective means of describing vegetation. To at least minimize the error, ecologists resort to quantitative measures, with statistical considerations. Although unavoidably time-consuming, these methods give workers a more realistic picture of the structure and dynamics of a vegetation.
(a) Density (D) - This is the count of the number of individuals within a series of randomly distributed sample areas (quadrats or plots). The method allows comparison of different areas and different species and is an absolute measure of abundance of the plants. Its disadvantages lies in the large amount of time involved in counting the individuals.
While density (often called crude or absolute density) is the number of organisms per unit total space or volume, much of the area or volume may not actually be colonized by the population. It is, therefore, more meaningful to speak of the number of organisms per unit of habitable space (or of specific or ecological density).

| Where | Di | = | density of species i; |
| ni | = | total number of individuals of species i; | |
| A | = | total area sampled. |
Often, it is more important to know whether a population is changing than to know its size at any one moment. In comparative studies, one generally wants to know the number of individuals relative to other populations. In such cases, relative species density, RD, is useful. It is the total number of individuals of a species expressed as a proportion (or percentage) of the total number of individuals of all species presents:

| Where | RDi | = | relative density of species i; |
| Di | = | density of species i; | |
| TD | = | sum of the densities of all the species. |
(b) Frequency (f) - Frequency is a measure of the chance of finding a species with any one throw of a quadrat in a given area. Thus, if Gracilaria has a frequency of 0.1, then it should occur once in every 10 quadrats examined:

| Where | fi | = | frequency of species i; |
| ji | = | number of samples (or smaller squares) in which species i occurs; | |
| k | = | total number of samples (smaller squares) taken. |
The method is done simply by noting whether a species is present or absent in a series of randomly placed quadrats (or in the smaller squares within a quadrat). For example, if 43 quadrats out of a total of 90 contain Gracilaria, the frequency is 43/90 = 0,48. This is the same as saying that the probability of finding the seaweed in the sample is 48 percent.
Greig-Smith (1975) proposed two convenient forms of frequency. namely, shoot and rooted frequency. The former considers a species as “present” only when any part of the plant thallus overlaps into the quadrat. The latter measure considers a species as “present” when it is actually rooted within the sampled area.
The only advantage of frequency as a measure of abundance lies in the ease and rapidity with which an area can be sampled. Values obtained using this method are dependent on the size of the quadrat used, and the size and spatial distribution of the individuals inside the area sampled.
The relative frequency, Rf, is the frequency of a given species expressed as a proportion of the sum of the frequencies for all the species:

| Where | Rfi | = | relative frequency of species i; |
| fi | = | frequency of species i; | |
| Tf | = | total of the frequencies of all the species. |
(c) Cover (C) - This is the proportion of the ground or the substratum occupied by the individuals or parts of the individuals of the species under consideration. It is an estimate of the area covered by the given species, expressed as a percentage of the total area, measured usually by taking a number of points from the sample area and determining at those points which species, if any, is covering the surface of the substratum. As this method involves visual estimates, it is prone to personal bias, as was discussed in relation to frequency ratings:

| Where | Ci | = | cover of species i; |
| ai | = | total area covered by species i; | |
| A | = | total habitat area sampled. |
The Domin Scale (discussed above) may be used to assign the cover values of the species.
The relative cover (RC) is the cover for a species expressed as a proportion of the total cover of all the species.

| Where | RCi | = | relative cover of species i; |
| Ci | = | cover of species i; | |
| TC | = | total cover for all species |
As a rough and overall estimate of the influence or importance of a plant species in the community, the importance value, IV, may be useful:
IVi - RDi + Rfi + RCi
(d) Standing crop biomass - This is the weight of existing species in a given area at any one time. Commonly, all the species Within a plot are collected, sorted, and dry-weighed. The result will give an estimate of the amount of matter produced by a species or a group of species in the area. Where there are large differences in the sizes of species present, standing crop biomass is an important measure of “abundance”:
B = (D) (TW/n)
| Where | B | = | the biomass; |
| D | = | density; | |
| TW | = | sum of the weights of the individual organisms in a sample; | |
| n | = | the number of individuals in the sample. |
Biomass for non-discrete; creeping (hence, non-individually countable) species is simply their absolute weights.
For organisms which vary greatly in moisture content, biomass should be expressed as dry weight rather than fresh weight. Biomass expressed as ash-free dry weight, nitrogen, carbon, or caloric content is useful in cases where the organisms vary in the amount of inorganic skeletal material.
(e) Growth rate (GR) - This is a measure of how well a species is growing in a given area. Measurements of growth rates in seaweeds depend on their modes of growth, i.e. for Ulva and Porphyra, it is best to measure” the change in area of an initial portion of a frond because they both exhibit diffused growth; for Laminaria, which grows by means of an intercalary meristem, growth rate is determined by getting the difference in the length of the frond after, some time interval:

| Where | GR | = | growth rate; |
| ΔP | = | mean of the change in the parameter used (area, length, weight, etc.) | |
| Δt | = | Mean change in time |
(f) Productivity (P) -This is the rate of organic matter production by organisms. In some cases, estimates of biomass may be useful to measure productivity:

| Where | P | = | gross biomass productivity; |
| B | = | standing crop biomass; | |
| T | = | turnover time |
Biomass is usually expressed as units of weight per area (or volume) of habitat, and productivity, as units of weight per unit area (or volume) per unit time. Hence, the turnover time is the number of time units (days, months, years) required to produce as much biomass as is present at the time of measuring the standing crop.
3.5.3 Determination of other ecological parameters
From the above basic ecological measures, others can be determined to reflect some of the structural aspects of the community not explicit in density, frequency, or cover. Indeces of species diversity, dominance and similarity or dissimilarity give better insights on the structure of the community in comparison with other communities or relative to a change in time. For example, the degree of influence of a fertilizer on the floral composition of a seaweed community may be assessed by monitoring the change in the diversity of the community. Physically-stressed habitats are generally less diverse than biologically-controlled ones.
(a) Species diversity - Species diversity is a measure of the number of species (the variety component) and the relative abundance of the individuals of each species (the evenness component). There are a number of diversity indeces proposed, each one with its own defined usefulness depending upon the nature of the community in question. Some of the more popular ones are: Shannon-Wiener Index (H'), Brillouin's measure (H), and Simpson's (D).
(b) Ecological dominance - This is a measure of the degree of control by a species or species groups of the energy flow and the environment of all the other species. A more popular index of dominance is given by Simpson (1949).
C = ∑(ni/N)2
| Where | C | = | dominance index |
| Ni | = | importance value for each species (i.e. number, weight, etc.); | |
| N | = | total of importance values. |
(c) Similarity/dissimilarity - Often after knowing the)seaweed flora of two or more communities, you ask the question: How similar (or dissimilar) are the communities in terms of the species present? Or you nay want to compare the species composition of the same community at two different times. In such instances, indeces of similarity or dissimilarity may be useful:
(i) Jaccard's coefficient (CCJ):

(ii) Sørensen's coefficient (CCS):

Where, in both (i) and (ii), c = number of species common to both : communities S1 and S2.
The index of dissimilarity is 1 - CCJ or 1 - CCS.
3.6 Objectively analyzing the data
Once the data are available, they should first be organized and systematized to facilitate the statistical procedures used in data analysis. It should be emphasized that it is only by means of statistical methods that: (i) characteristics of sets of, data can be quantitatively described and summarized; (2) conclusions about large sets of data, using only samples of them, can be drawn; and (3) relationships between pets of data, established. Hence, in the present paper, simple statistical manipulations will be frequently encountered but for no other reason than the fact that a good ecological work requires them.
An understanding of some basic statistical concepts is needed at this point:
Statistical population - the entire set of data about which we wish to draw conclusions. (For example, a population of Gracilaria at Calatagan).
Statistical sample - a portion of the statistical population.
Parameter - in statistics, it is a measure that describes or characterizes an entire population of data (For example, density or frequency of Caulerpa).
Statistics - descriptive measures derived from sample data taken from the population (For example, mean and median).
3.6.1 Descriptive statistics
Although we cannot directly measure a parameter of a population, we can describe, for example, the density of a seaweed population by using the:
mean - this is a measure of the central tendency of a population and is computed as:
X = ∑X/n
| Where | X | = | sample mean; |
| ∑X | = | Sum of all values of × in the sample; and | |
| n | = | number of data in sample. |
The sample mean is a reasonable estimate of the population mean only when the former is obtained at random -from the entire population.
Median - this is the middle measurement in a ranked data. If there are an even number of data, the median is the mean of the two middle measurements.
Range - a measure of how variable the gathered data are, the range is simply the difference between the largest and the smallest measurement. The big disadvantage in using the range to describe dispersion of sample data is that it tends to underestimate the population range.
Standard deviation (s) - this is a measure of how the data are dispersed relative to the mean. For this reason, it becomes very useful in statistics:

| Where | s | = | sample standard deviation; and |
| s2 | = | sample variance. |
Variance - this variance is:
S2 = SS/DF
Where SS (“sum of squares”) = ∑(X - X)2; and
DF(“degrees of freedom”) = n - 1.
Standard error - we often ask the question: How precise is our estimated mean? If we have replicates of, for example, the cover of Caulerpa taken from a population, each sample will have a different mean and how the sample means vary from each other can be measured by the standard deviation of the mean or standard error (SE).

| Where | s | = | standard deviation; and |
| n | = | number of data in the sample. |
Confidence interval - with a known standard error, we can set a range, with a stated level of confidence, within which the population mean lies:
(1 - 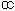 ) confidence interval for population mean, μ = X ± tSE
) confidence interval for population mean, μ = X ± tSE
| Where | 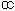 | = | significance level: |
| X | = | sample mean; and | |
| SE | = | standard error. |
The value of t is obtained from a statistical distribution known as “Students' t”, a portion of which is given below:
| DF | 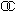 = 0.10 = 0.10 | 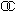 = 0.05 = 0.05 | 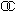 = 0.02 = 0.02 | 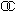 = 0.01 = 0.01 |
| 1 | 6.31 | 12.71 | 31.82 | 63.66 |
| 2 | 2.92 | 4.31 | 6.96 | 9.92 |
| 3 | 2.35 | 3.18 | 4.54 | 5.84 |
| 4 | 2.13 | 2.78 | 3.75 | 4.60 |
| 5 | 2.01 | 2.57 | 3.36 | 4.03 |
| 6 | 1.94 | 2.45 | 3.14 | 3.71 |
| 7 | 1.89 | 2.36 | 3.00 | 3.50 |
| 8 | 1.86 | 2.31 | 2.90 | 3.36 |
| 9 | 1.83 | 2.26 | 2.82 | 3.25 |
| etc. | etc. | etc. | etc. | etc. |
Selecting Statistical Sample Size
In general, the greater the value of n = (number of data in the sample), the smaller the amount of error and the more precise the estimate of the population mean. This is evident from the formula for SE above. From this relationship, we can determine the number of data (n) required to estimate the population mean with a specified precision.
3.6.2 Comparing statistical populations
Phycologists may often ask the question: Are the mean seaweed biomass of, for example, two apparently similar portions of the shore, the same? Are they the same in two different seasons? The community coefficients (CCJ and CCS) discussed above give only the relative degree of similarity between the communities. They do not tell us whether that difference is a result of habitat - specific conditions or of other factors.
The more common tests to compare statistical populations-are: two-sample (or t -) testing, multisample testing (analysis of variance, ANOVA) and non-parametric testing.
The first two are applicable only in instances where the populations being compared have equal variances and each population is composed of data which conform to the “normal distribution”. For example, lengths, weights, heights and rates can be compared using t-testing and ANOVA. But percentages or proportions, densities, pH and other data measured on a nonlinear scale should not be subjected to the above tests. Instead, they are analyzed using non-parametric or distribution-free methods.
3.7 Interpreting and drawing conclusions from the data
This aspect of the scientific study is almost solely dependent on the experience and knowledgeability of the worker. Relevant information available from the literature and data obtained from measurements of environ-mental variables are very useful at this point. But they are so only to the extent of supporting and/or supplementing the primary findings just obtained from the study. So long as the interpretation of results and the conclusions drawn from the data have conformed to the steps outlined above, in a form that is easily comprehensible, and they answer the basic questions posed before the survey was conducted, it is fairly reasonable to assume that a “good job” is almost done.
4. REFERENCE
Brower, J.E. and J.H. Zar. 1977 Field and laboratory methods for general ecology. Wm. C. Brown Co.* Iowa: 194p.
by
Gavino C. Trono, Jr.1
1. INTRODUCTION
Due to the maritime nature of most of the Asian countries, it is inevitable that a significant portion of the population is located along ., the coastal areas. The lives of the people are therefore intimately associated with the sea and the marine resources. These coastal areas are located in far-flung places away from urban and industrial centres.
The coastal areas of many countries in Asia are characterized by well-developed coral reefs which abound in fishery resources. However, the rapid increase in population in these areas as well as the increasing demands for fishery products by urban populations are exerting tremendous pressure on the productivity of these resources. The fishery resources in many of the coastal areas are over-exploited. Alternative source of food and livelihood must be found to improve the lot of the generally poor coastal inhabitants.
Among the coastal resources whose potentials have not yet been tapped is the seaweed fishery. Many species of seaweeds of high economic value are found in shallow reef areas. The principal genera of seaweeds presently utilized in limited scale in Asia are listed in Table 1. The farming and production of seaweeds along the coastal areas offer a good alternative as source of food and cash for the coastal inhabitants.
The farming of Eucheuma in the Philippines is a good example to illustrate the economic benefits which could be derived from the development of the seaweed resources in Asia. Several thousand coastal inhabitants in the Southern Philippines and in Central Visayas are presently engaged in Eucheuma farming. Approximately, 15 000 MT tons of dried Eucheuma valued at 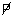 57 million2 were produced in 1980.
57 million2 were produced in 1980.
2 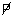 sign for Philippine pesos; 7.65 pesos - US$1.00.
sign for Philippine pesos; 7.65 pesos - US$1.00.
2. PRESENT STATUS OF PRODUCTION
Except for a few species which are presently being farmed utilizing available fanning techniques, e.g. Eucheuma striatum (cottonii type), E. spinosum (spinosum type), Caulerpa lentillifera and Gracilaria verrucosa, the bulk of the present seaweed production in Asia comes mainly from the harvesting of wild crops.
Production from wild crops is unreliable and is dependent on the natural growth of the species and the harvest rate exerted on the local stocks. Post-harvest methods used are generally antiquated resulting to poor quality of the produce. In addition, the absence of a natural management programme for these species often results to the depletion and/or destruction of natural stocks. In contrast, production of species through farming is reliable and highly labour-intensive, thus offering more job opportunities and the use of the local manpower resources is maximized. In addition, farm-produced seaweeds are generally of higher quality due to selection of stock and the application of improved post-harvest handling methods.
3. UTILIZATION OF SEAWEEDS AND SEAWEED PRODUCTS
Many seaweed species are utilized as food by coastal inhabitants in many Asian countries. These are usually eaten as vegetable and prepared in various ways. Except for a few species, seaweeds are of low nutritional value due to their low protein content and generally hard-to-digest carbohydrates. However, they are good source of minerals and probably vitamins. Thus, these are good food supplements but cannot totally replace the staple foods such as rice, fish and other cereals.
The high commercial potentials of the seaweeds is due to their importance as raw materials in the manufacture of phycocolloids such as agars, carrageenans and alginates which are applied in many industries. However, due to the lack of processing technology the bulk of seaweeds produced in Southeast Asia are sold in the international market in unprocessed form (dried). These countries then buy the processed product from the foreign processors or suppliers at a much higher price. At present, only limited amounts of agarophytes are processed in Southeast Asian countries to produce crude agar which is available in local markets in the form of dried agar bars.
4. FUTURE NEEDS OF THE SEAWEED RESOURCES
The potentials for developing the seaweed resources in Asia is very good. Many commercially important species presently produced from unmanaged wild crops can be significanzly improved through the application of conservation and development programmes. Many other species have yet to be discovered and their commercial values determined through natural product screening work. In addition, more than enough suitable reef areas which are at present hardly utilized are available for wild crop production or mariculture.
There is, however, the need to improve the capabilities of Asian countries in terms of technical expertise and financial support before research and development programmes can be implemented in the region. Foreign assistance will be necessary in this regard.
Table 1. Principal seaweed genera of economic potentials in Asia
| Country | Genera | Uses | Status of production | |
| 1. | Philippines | Caulerpa | food | Pond culture and wild crops |
| Codium | food | Wild crops | ||
| Sargassum | alginate | Wild crops | ||
| Porphyra | food | Wild crops | ||
| Gelidiella | agar | Wild props | ||
| Gracilaria | agar, food | Wild crops | ||
| Eucheuma | carrageenan, food | Mariculture | ||
| Hypnea | food | Wild crops | ||
| 2. | Indonesia | Gracilaria | agar, food | Wild crops |
| Eucheuma | carrageenan | Wild crops | ||
| Mariculture | ||||
| Gelidiella | agar, food | Wild crops | ||
| Hypnea | carrageenan, food | Wild crops | ||
| Caulerpa | food | Wild crops | ||
| Acanthophora | food | Wild crops | ||
| 3. | Singapore | Eucheuma | Carrageenan | Wild crops |
| Gracilaria | agar | Wild crops | ||
| Sargassum | alginate | Wild crops | ||
| 4. | Brunei | Gracilaria | agar | Wild crops |
| 5. | East Ma4aysia | Porphyra | food | Wild crops |
| Sargassum | alginate | Wild crops | ||
| Eucheuma | carrageenan | Wild crops | ||
| Caulerpa | food | Wild crops | ||
| Gracilaria | agar | Wild crops | ||
| 6. | West Malaysia | Gracilaria | agar | Wild crops |
| 7. | Thailand | Gracilaria | agar | |
| Porphyra | food | Wild crops | ||
| 8. | Vietnam | Gracilaria | agar | Wild crops |
| Sargassum | alginate | Wild crops | ||
| 9. | Hong Kong | Sargassuum | alginate | Wild crops |
| Porphyra | food | Wild crops | ||
| 10. | Taiwan, China | Gracilaria | agar | Pond culture |
| Porphyra | food | Wild-crops(?) | ||
| 11. | Sri Lanka | Gracilaria | agar | Wild crops(?) |
| Porphyra | alginate | Wild crops | ||
| 12. | India | Gracilaria | agar | Wild crops(?) |
| Mariculture | ||||
| Gelidiella | agar | Wild crops | ||
| Sargassum | alginate | Wild crops | ||
| Hypnea | carrageenan | Wild crops | ||
| 13. | Burma | Gracilaria | agar | Wild crops |
| Gelidium | agar | Wild crops | ||
| Sargassum | alginate | |||
| 14. | Pakistan | Gracilaria | agar | Wild crops |
| Gelidium | agar | Wild crops | ||
| Hypnea | carrageenan | Wild crops | ||
| Porhyra | food | Wild crops |
5. REFERENCES
Doty, M.S. 1977 Seaweed resources and their culture in the South China Sea Region. SCS/77/WP/60: 19pp.
Michanek, G. 1975 Seaweed resources of the Ocean. FAO. Fish. Tech. Paper No. 138
Naylor, J. 1976 Production, trade and utilization of seaweeds and seaweeds products FAO Fish. Tech. Paper, No. 159.
by
Gavino C. Trono, Jr.
The “non-farmable” species as used in the present context, are those which by the very nature of their small size, slow growth and regenerative capacities and/or determinate growth cycles do not easily lend themselves to the conventional way of farming through mass production through the use of cuttings or spores, e.g. Gelidiella acerosa, Gelidium spp., Sargassum sp., and Acanthophora. Their production depends primarily on the availability of naturally produced stocks as influenced by harvest pressures during the preceding season. Production of some of the species is highly seasonal depending on their growth cycles as influenced by environmental conditions. Their harvestible stocks are also significantly controlled by monsoons.
Because their growth cycles are highly dependent on the environmental conditions in their habitat and to a large extent to the degree by which these are influenced by man's exploitive activities,' their production, . therefore, is highly unreliable. The need to manage and conserve their natural stocks is of prime importance in order to assure to a certain extend their continuous production as well as to prevent over-exploitation.
The design of a sound management and conservation scheme for non-farmable species depends primarily on the availability of information on the various aspects of their biology, e.g. reproduction and growth cycles, growth rates, their regeneration and recruitment capacities, and their production potentials. The above information are necessary in the formulation of guidelines for the management of the natural stock of the target species. These information can provide answers to questions such as where the species is abundant, how much to harvest per unit area, when to harvest, how many times can the stocks be harvested in one season, what kind of harvest method is best for the species. Furthermore, production can be safely forecasted. These available information is most important in quoted contracts which may be entered into by the farmer, fisherman, or exporter. The gathering of these basic information on the species to be managed requires basic skills in methodologies for field sampling and data gathering. Instructions on these methodologies are included in this training course for this purpose.
Thus, it is of prime importance that any plan to exploit natural stocks of seaweeds must be preceded by intensive biological studies to determine seasonality in biomass production, reproduction, regeneration and recruitment. These information are necessary in determining the best possible time of harvesting and amount of harvestible stocks.
Production of non-farmable species can be enhanced by the application of some agronomic techniques with the primary aim of protecting the natural stocks from over-exploitation. The application of harvest techniques which are the least destructive is one way of assuring the fast recovery of stocks. For instance, harvest by hand picking (pulling) is more destructive than prunning. The removal of the whole plant from the substratum by hand-picking reduces the capacity of the stocks to regenerate. Renewal of stocks through regrowth from basal portions left after prunning is very much faster than recruitment of new thalli from spores.
The removal of unwanted species (weeding) may enhance the growth and development of target species through eradication of competitors for space, light, and nutrients. The clearing of the substrate also enhances the opportunity for recruitment by spores. Studies have shown that recolonization of bare substrates results to the increase in population density of the target species.
The eradication of grazers in reef areas being managed for wild crops contributes to the enhancement of production in reef areas. Sea urchins, starfishes, and fishes like siganids are well-known seaweed grazers whose destructive effects on seaweed crops can be minimized.
The harvesting of seaweed crops from natural stocks is labour-intensive. Seaweed gathering for cash crops, therefore, offers an opportunity to optimize the utilization of the minimally used labour force in coastal areas. Methods being employed in the harvesting of natural stocks vary depending on the species, its size, abundance and the habitat where these are found. In tropical areas, most of the wild crops are found in shallow rocky portions of the reef, or in shallow bays. In reef areas where harvestible crops are closely associated with other species, selective harvesting through hand-picking and/or prunning are the most common methods of cropping. In shallow bays, the gathering of Gracilaria is done by hand, or with the use of rakes. In some instances of semi-mechanized method is employed utilizing trawl-like equipment attached to a slow-moving motorized bancas. In deeper areas, diving and hand-picking or the use of prunning tools are employed. SCUBA is a convenient equipment now used in some countries for gathering wild stocks in deeper areas.
In wave-exposed areas where hand-picking is hazardous, the gathering of seaweed crops is mostly dependent on drift materials which accumulate on the shore especially after some heavy surfs.
by
Gavino C. Trono, Jr.
1. INTRODUCTION
Seaweeds and other algae have been traditionally grown in fishponds as £i8h food. Some of the common species of seaweeds grown in ponds are Enteromorpha sp., Gracilaria verrucosa, Cladorphora sp. and Ulva sp. Wild crops of these species are gathered and placed in ponds as supplementary food for milkfish. In addition, large quantities of these seaweeds are utilized as organic fertilizer in ponds.
Recently, the increase in demand for seaweeds as raw materials for the manufacture of agar or as fresh vegetables which wild crops cannot supply has encouraged the pond culture of certain species such as Gracilaria and Caulerpa. However, ponds being an enlcosed system has certain disadvantages, i.e. ecological parameters such as salinity and water temperature go through a wide range of variation. Thus, only few species which are highly adapted to such changes can be cultured. In spite of their apparent adaptability to pond conditions, the pond cultured species still show seasonality in their growth and production. For instance, Caulerpa lentillifera production still suffers from much lowered salinity during months of heavy rainfall while production of Gracilaria verrucosa is minimal during the dry seasons due to high salinity regimes in ponds.
The pond culture of seaweeds appear to be a good alternative in many areas where the culture of fish and shrimps have become very unproductive. Many of these abandoned ponds are good areas for the culture of seaweeds. Thus, there is a good prospect for maximizing the utilization of these areas through seaweed culture.
2. CULTURE OF GRACILARIA
At present, the production of Gracilaria in fishponds in the Philippines is for other purposes, e.g. as fish food rather than as cash crops. There is no established technology for its monoculture or as polyculture with other products such as fishes and shrimps. In the Asian region, only Taiwan, China and Vietnam have developed the technology for its culture as “cash crops”.
The. successful production of Gracilaria in ponds requires the maintenance of a number of ecological parameters most favourable for the species being cultured. These can be attained through proper water management. The following information on these requirements are based on the Taiwan experience:
| Salinity | = | 18–30 ppt; 24 ppt optimum |
| Water temperature | = | 20–25°C |
| PH | = | 6–9 |
| Depth | = | 30 cm during non-sunny months 60–80 cm during sunny months |
| Substrate | = | sandy loam |
The -culture sites (ponds) should be located in protected areas where a constant supply of freshwater is available. The freshwater is needed in-maintaining the favourable salinity range for the seaweeds.
The species of Gracilaria most adapted for pond culture in Taiwan and Vietnam is G. verrucosa because of its ability to adapt to a wide range of environmental conditions in ponds. The propagation of this species is through the use of cuttings which are applied at the rate of 500 kg per hectare. Planting is done through broadcast method.
Pond management is an important aspect in the culture of Gracilaria. The regular changing of pond water is important in maintaining the nutrient, salinity and water temperature best suited for the species. The presence of other seaweed species in ponds is one of the problems which affects production and quality of the produce. Weeding of these obnoxious species is important to minimize “weeds” in ponds. In Taiwan, the ponds are seeded with Tilapia to graze on the weeds/epiphytes. The fishes are removed once the weeds are placed under control to prevent the fish from grazing on the Gracilaria.
The application of both organic or inorganic fertilizers is also being done in Taiwan to accelerate the growth of plants.
Harvesting is done by hand or with the use of scoop nets. Both mono-culture and polyculture of Gracilaria with fish or shrimp are practiced in Taiwan. Investment returns for monoculture is 50 percent while polyculture is 11 percent.
3. POND CULTURE OF CAULERPA
In spite of the successful culture of Caulerpa in ponds in Mactan, Cebu in the Visayas, Central Philippines, which started several years ago, there is no published information on its methodology or feasibility as a business venture. The unavailability of such information is one of the major constraints which hinders the possible transfer of this culture technology to other areas. The present high demand for Caulerpa in the local markets and the inadequate supply have caused the price of the seaweed to increase.
The potential of Caulerpa as an export item is very good. A sizeable quantity is presently exported to Japan, both in its fresh state or dehydrated form. Expanded production programme for this genus can easily be met by the utilization of the abandoned or unproductive fishponds.
The culture of Caulerpa in ponds requires the maintenance of certain ecological parameters for its proper growth and development. The farm site must be protected from the adverse effects of wind and waves. The substrate must be clay-loam. Because Caulerpa is eaten as fresh vegetable, it is necessary that the farm site must be located in a place which is free from pollution.
Caulerpa is a stenohaline marine alga. It requires a salinity of 30–32 ppt for optimum production. The depth of the pond may vary from 60 – 100 cm depending on the clarity of the water. C. lentillifera presently being cultured is known to be sensitive to full sunlight. The depth of the water found to be optimum is that depth where the plants can be barely seen from the surface of the water. Water temperature should be maintained at approximately 27–30°C for optimum production. Temperatures 3–5° higher than this range has adverse effects on growth.
Water management in ponds is an important aspect to consider. The ease in water management can be attained through careful site selection and pond construction. The pond level must be approximately at the 0 datum level so that the pond water can be frequently changed to maintain optimum water temperature, salinity and pH (7–8 as optimum). pH higher than this results to stunted growth and increase in toughness of the thalli which reduces the quality of the seaweed.
There is no precise information on the amount of seedstock required to plant a hectare farm. However, the results of our initial studies have shown that 50 kg of seedstock is enough to seed a 625 m2 pond (or approximately 1.5 tons per hectare). However, because of the high price of seed-stocks,- more studies on this aspect is being pursued to determine the optimum amount necessary per unit area.
Planting is by broadcasting. Seedstocks are broadcasted as evenly as possible in the pond. This method, though practical, had been found to have certain disadvantages, i.e. the planting materials tend to float and do not sink immediately to the bottom. Thus, these materials may be carried to certain portions of the pond by water movement caused by wind. An innovative method was developed by farmers to correct this problem. A handful of mud, preferably clay, is molded at one end of the cuttings to serve as sinker. These are then thrown evenly into the pond.
The “reseeding” of the pond is not necessary if enough seedstock are left during harvest time. Under optional conditions, the plants can be harvested in about 2–3 months time. The plants are ready for harvesting when these have already evenly covered the pond bottom.
Fertilizer be applied to hasten growth of the plants. Application must be done when the newly planted seedstocks are already showing signs of new growth . The application rates of fertilizer has not yet been determined. One of the important considerations in fertilization is that the method of application must provide for the slow release of dissolved fertilizer in the water to avoid wastage. Our initial experience show that one-half kilogram of inorganic fertilizer can be applied several times by wrapping this in several layers of gunny sock or some other materials. The fertilizer is then tied to a stake in the middle of the pond at a height were the fertilizer is half submerged in the water. The fertilizer is then removed after one to two hours of exposure. This application may be repeated. Application is done right after the water in the pond has been changed. There is no complete information available on the economic aspects of the Caulerpa farming. However, it is very apparent that the fanning of this seaweed is a lucrative business in Mactan Island, Cebu, Philippines. The farm area has increased significantly since farming started in the island. About a dozen pond owners have converted their fishponds into Caulerpa ponds.
4. REFERENCES
Chang, C.Y. 1916 The economic aspects of Gracilaria culture in Taiwan. Aquaculture 8: 1–7.
Hortsmann, U. 1978 Nearshore Macroalgae culture in topical developing countries. The Philippine Scientist 15: 67–75.
Sotto, F. 1978 The culture of Caulerpa racemosa in Kalawisan, Mactan Island, Cebu, Philippines: A potential for the seaweed industry. The Philippine Scientist 15: 109–111.