L.K. Menin and D.M. Njarui
National Dryland Farming Research Centre, Katumani
P.O. Box 340, Machakos, Kenya
W.M. Beattie, B.A. Keating and R.K. Jones
ACIAR/CSIRO Dryland Project
P.O. Box 41567, Nairobi, Kenya
Introduction
Materials and methods
Results
Discussion
Acknowledgements
References
Appendix 1. Legume-evaluation: Accession list.
Abstract
The adaptation of 160 legumes of various growth forms is being studied at four sites in the semi-arid, mid-altitude districts of Machakos and Kitui in Kenya. The sites cover an altitude range from 900 to 1600 m and mean annual rainfall range from 600 to 1000 mm. Establishment is by direct-seeding using a minimum tillage approach. Phenological characters, susceptibility to pests and diseases, and growth vigour are measured monthly, and dry-matter production annually in row-plot experiments. Seasonal yields of dry matter and nitrogen are also measured in companion sward experiments using a "best bet" sub-set of the collection.
A number of legumes which are well-adapted to the environments have been identified after three growing seasons and appear to have potential for a variety of uses in the local farming systems.
Agriculture in the semi-arid midland regions of eastern Kenya is characterised by very low capital inputs, low outputs (even in good seasons) and a high frequency of seasons in which production of staple foods is insufficient for local needs. Rapid increases in rural population densities and in the intensity of cultivation, fuelwood harvesting and grazing have resulted in alarming rates of soil fertility depletion and soil erosion. While rainfall is the principal limiting resource, it is usually closely followed by soil fertility, hence the need to examine the role of legumes in the cereal-based farming systems of the regions as an alternative to the use of expensive nitrogen fertilizers.
FAO/GK/NORAD projects since 1974 (Wilson, 1976; Van Soest, 1986; Ibrahim, 1981; Chabeda, 1976) have made extensive collections of forage legumes within Kenya and evaluated them at four sites - Kitale, Kiboko, Katumani and Mtwapa along with some introduced material. However, much of the evaluation work is unpublished or is of a semi-quantitative nature, and the full potential of forage and browse legumes in Kenya's bimodal semi arid areas remains little understood. In addition, many new species and accessions have become available from more recent plant collecting and evaluation activities in other parts of the world, and this material required testing in Kenya.
The objectives of the research programme reported here are:
1. to assemble an extensive seed collection of forage and browse species with potential adaptation to semi-arid areas of Kenya,2. to assess the adaptation of this material in a series of experiments to a range of climatic conditions in the districts of Machakos and Kitui,
3. to select a nucleus of widely-adapted legumes that might fit into the varied farming systems in these districts, and to assist agronomists and animal production scientists in further evaluating this material in "on-farm" experimentation.
The work involves close collaboration between staff of the Kenya Government's Ministry of Agriculture and staff of the Australian Dryland Farming Project (ACIAR Project 8326) which is being implemented by the CSIRO Division of Tropical Crops and Pastures.
Legumes
A total of 167 accessions from 23 genera are under evaluation. Details of ecotypes/species, Commonwealth of Australia Plant Introduction numbers, Kenya Introduction numbers, origin (where known) and project sites planted are contained in Appendix 1.
Field sites
The experiments were undertaken at four fenced sites as follows:
1. Katumani NDFRS, altitude 1600 m, mean annual rainfall 717 mm, mean annual temperature 19.6°C, soil type: chromic luvisol, pH 6.5.2. Kiboko RRS, 975 m, rf 595 mm, mean temperature 25.7°C, rhodic Feralsol, pH 5.8.
3. Mua Hills, 1650 m, rf 1179 mm, estimated mean temperature 19.0°C, red sandy earth, pH 7.0.
4. Ithookwe (Kitui), 1160 m, rf 1080 mm, mean temperature 22.5°C, red sandy earth, pH 5.8.
Experimental Design and Layout
Row plot experiment. The experiments were laid in a randomised complete block design with 104 accessions and 3 replicates. Plots are 6.75 m² and consist of two rows each 2.25 m long and rows 1.5 m apart. Replicates run along the contour. One of each pair of rows received a single dressing of a basal fertilizer. The other row was unfertilized.
Sward experiments. For each season of planting, the experiments were arranged as randomised blocks with 20 accessions and three replicates. Individual plots were 16 m². Seed was not broadcast over the entire plot but distributed along disturbed rows 0.5 m apart. All plots received a basal fertilizer dressing at establishment.
Seed Preparation
Row plot experiments. Seed viability was determined by germinating scarified seed on moist filter pads in petri dishes. Approximately 50% of all seeds/plot were scarified by sandpaper or scalpel. Inoculation with the requisite rhizobia in a gum arable/distilled water solution was made a few days prior to planting. Inoculated seed was kept under refrigeration.
Sward experiments. Seed preparation was similar to that for row plots except that 100% seed scarification was attempted, and the seeding rate was adjusted to give similar numbers of viable seeds per plot (Table 2).
Land Preparation, Sowing and Seedling Establishment
Land preparation was minimal except for Ithookwe (site 4) which was ploughed just prior to planting. Vegetation was cut back and burned off during the dry season and line rows pegged out. A small chisel plough attached to a two-wheel Honda mini-tractor marked a 4 cm deep furrow along each planting row. At the onset of rains, when weed seedlings had emerged, the furrows were dusted with Aldrin 2.5% @ 20 kg/ha. Legume seed was then hand-sown, lightly covered and firmed with soil. Two days later or just prior to legume germination the planted rows were sprayed with Roundup herbicide 2 l/ha using a knapsack sprayer fitted with a fan nozzle. Subsequent row weeding was done by hand or with a rope wick containing Roundup.
Data Collection/Harvesting
Row plot experiments (Table 1). Agronomic and phonological characters recorded monthly from the second month after sowing included plant population and development (vegetative, flowering and seeding), height, date of anesthesia, insect and disease incidence and a visual bulk rating on a one-five scale. In early October 1986, after 11 months of uninhibited growth, a measured length of average plant material in each row plot was cut at 5 cm above ground level (5 cm from the crown for trailing legumes), oven dried at 60°C and weighed. Sample weight was converted to yield (kg/ha) by the formula
Sample experiments. Plant measurements similar to those given above were made except that for visual bulk ratings. Harvesting entailed cutting two randomised 1 m² quadrats 5 cm above ground level from each plot three times per year. The first harvest was in late January, 1986 (end of short rains), the second in early June, 1986 (end of long rains) and the third in late September, 1986 (end of dry season). The latter harvest also included measurements of regrowth from the first harvest. Cut material was oven-dried at 60°C, weighed and subsampled for later N analysis.
Fertilizer Applications
Two weeks after legume seedling emergence a basal fertilizer was hand broadcast along one row of the row plots to a width of 1.5 m and evenly broadcast over the swards. Rates in kg/ha were: triple superphosphate 200, muriate of potash 41.7, dolomite 250, manganese sulphate 15, borax, zinc sulphate and copper sulphate 10, respectively and sodium molybdate 0.36. The swards received the same mixture broadcast over the entire plot.
Methodology for Experiments with Leucaena Accessions
At Katumani, a collection of Mexican high altitude Leucaena accessions is being tested quantitatively against a suite of commercial cultivars including cv Cunningham, cv Peru and K8. Adaptation is progressively monitored throughout each year by measuring shoot extension growth relative to soil moisture content and air temperatures. Destructive harvests are made twice and four times per year for wood, stem and leaf yield.
Row Plot Experiments
Data from 16 accessions which performed well at Site 1 (Katumani), Site 2 (Kiboko) or both sites are presented in Table 1. Of the annuals, the short season Macrotyloma africanum with densely twining fine stems, had high resistance to insect and disease, good seed production and an excellent seedling regenerative capacity. Another trailing legume Vigna unguiculata CI60452 was more strongly persistent at both sites than other entries from that genus. Alysicarpus rugosus established well and had the highest edible dry matter (EDM) (1938 kg/ha) at the end of the evaluation period at Site 1. The two Stylosanthes hamata lines, cv Verano and K14363, are morphologically similar, but the latter persisted later into the dry season. Both accessions and the late flowering S. humilis are particularly susceptible to termites. Lablab purpureus cv Rongai grew vigorously at both sites and gave high visual bulk ratings. It flowered profusely, but seed production was reduced by the depredations of flower-eating beetles Mylabris and Coryna sp.
The selected perennial legumes (Table 1) have persisted strongly at Site 1 but only Desmanthus virgatus, S. scabra cv Fitzroy cv Seca and S. guianensis Alupe 'C' survived the, harsher environment at Site 2. These Stylosanthes accessions yielded >4 tonnes EDM/ha at Katumani and appear particularly well adapted at that site. The semi-erect shrub legume Desmanthus virgatus K14456 was slow to establish but showed promise later in the experiment at both sites. Neonotonia wightii was also slow to establish but grew vigorously after the second season. Cassia rotundifolia cv Wynn flowered very early and continued to produce seed throughout the year. It has persisted strongly at Site 1 with useful seasonal seedling regeneration. Desmodium intortum cv Greenleaf is susceptible to termites and while quite vigorous during seasonal rains only just survived the long dry season at Site 1.
Table 1. Field data from 16 selected forage legumes at two sites (Est. 1.11.85).
|
Accessions |
PBR |
PBR |
Max. ht (cms) |
Anesthesis (days) |
Seed Production |
Pet disease |
EDM yield (kg/ha) |
|||||||||
|
Site |
Days |
Site |
Days |
Site |
Site |
Site |
Site |
Site |
Site |
1 |
2 |
1 |
2 |
Site |
Site |
|
|
1 |
|
2 |
|
1 |
2 |
1 |
2 |
1 |
2 |
|
|
|
|
1 |
2 |
|
|
Alysicarpus K14384 |
3.6 |
180 |
3.0 |
120 |
30 |
20 |
80 |
140 |
2 |
2 |
0 |
0 |
0 |
0 |
938 |
- |
|
Lablab purpureus cv Rongai |
4.3 |
90 |
5.0 |
60 |
55 |
60 |
110 |
200 |
2 |
2 |
2 |
0 |
0 |
0 |
- |
- |
|
Macrotyloma africanum K14348 |
3.5 |
90 |
3.2 |
90 |
10 |
15 |
55 |
80 |
3 |
2 |
0 |
0 |
0 |
0 |
- |
- |
|
Stylosanthes hamata cv Verano |
5.0 |
210 |
4.0 |
180- |
20 |
25 |
60 |
60 |
3 |
3 |
3 |
0 |
0 |
0 |
678 |
- |
|
Stylosanthes hamata K14365 |
4.8 |
210 |
4.3 |
210 |
15 |
15 |
60- |
60 |
3 |
3 |
3 |
1 |
0 |
0 |
820 |
- |
|
Stylosanthes humilis K14368 |
4.8 |
210 |
4.1 |
210 |
10 |
15 |
150 |
170 |
3 |
3 |
2 |
0 |
2 |
0 |
362 |
- |
|
Vigna unguiculata CPI 60452 |
3.2 |
150 |
4.0 |
60 |
15 |
25 |
60 |
60 |
2 |
2 |
1 |
0 |
1 |
0 |
- |
- |
|
Cassia rotundifolia cv Wynn |
5.0 |
210 |
3.0 |
180 |
20 |
20 |
50 |
30 |
3 |
2 |
1 |
1 |
0 |
0 |
851 |
- |
|
Desmanthus virgatus K14456 |
3.1 |
300 |
2.6 |
180 |
25 |
25 |
120 |
140 |
2 |
2 |
1 |
0 |
0 |
0 |
720 |
- |
|
Desmodium intortum cv Greenleaf |
3.5 |
240 |
1.6 |
90 |
40 |
15 |
210 |
- |
2 |
0 |
2 |
0 |
0 |
0 |
1198 |
- |
|
Neonotonia wightii K2366 |
4.8 |
210 |
3.0 |
210 |
30 |
20 |
200 |
210 |
3 |
1 |
1 |
1 |
0 |
0 |
1551 |
309 |
|
Stylosanthes fruticosa K14426 |
4.5 |
210 |
2.6 |
270 |
25 |
15 |
110 |
90 |
3 |
2 |
1 |
0 |
0 |
0 |
2015 |
415 |
|
Stylosanthes guianensis cv Graham |
4.3 |
210 |
3.6 |
210 |
30 |
20 |
100 |
60 |
3 |
3 |
1 |
0 |
0 |
0 |
1407 |
551 |
|
Stylosanthes guianensis Alupe 'C' |
5.0 |
210 |
4.5 |
210 |
45 |
40 |
(90) |
180 |
2 |
1 |
1 |
1 |
0 |
0 |
4175 |
|
|
Stylosanthes scabra cv Fitzroy |
5.0 |
210 |
4.0 |
210 |
45 |
35 |
100 |
90 |
3 |
3 |
1 |
0 |
0 |
0 |
4112 |
|
|
Stylosanthes scabra cv Seca |
5.0 |
210 |
4.0 |
210 |
60 |
50 |
100 |
90 |
3 |
3 |
1 |
0 |
0 |
0 |
4127 |
|
Site 1: Katumani NDFRS
Site 2: Kiboko R.R. Station
RPBR : Peak Bulk Rating
Seed Production: 0 = nil, 3 = good
Pest/Disease: 0-1, 3 = severe
EDM yield: at 11 months.
- Not available
|
|
1985 |
1986 | ||||||||||
|
Rainfall (mm) |
Oct |
Nov |
Dec |
Jan |
Feb |
Mar |
Apr |
May |
Jne |
Jul |
Aug |
Sept |
|
Site 1 |
85 |
57 |
137 |
63 |
- |
43 |
163 |
100 |
7 |
- |
- |
- |
|
Site 2 |
|
|
|
|
|
|
|
|
|
|
|
|
Table 2. List of accessions used in sward studies.
|
|
Present in planting |
||||
|
number |
Mean sowing rate kg/ha |
||||
|
1 |
2 |
3 |
4 |
|
|
|
Alysicarpus rugosus CPI 52351 |
* |
* |
* |
* |
4.3 |
|
Alysicarpus rugosus CPI 30034 |
|
|
* |
|
4.3 |
|
Cassia rotundifolia Q10057 |
* |
|
|
|
5.2 |
|
Cassia rotundifolia cv Wynn |
|
* |
* |
* |
5.2 |
|
Centrosema pascuorum cv Cavalade |
* |
* |
* |
|
26.1 |
|
Centrosema pascuorum CPI 65950 |
* |
* |
|
|
26.1 |
|
Centrosema schottii CPI 82271 |
* |
* |
|
|
26.1 |
|
Centrosema schottii CPI 76010 |
* |
* |
|
|
26.1 |
|
Centrosema virginianum CQ 2748 |
|
|
* |
* |
26.1 |
|
Clitoria ternatea CPI 48337 |
* |
* |
* |
* |
43.8 |
|
Clitoria ternatea CPI 47187 |
* |
* |
* |
* |
43.8 |
|
Clitoria ternatea CPI 49963 |
* |
* |
* |
* |
43.8 |
|
Desmanthus virgatus CPI 40071 |
* |
* |
* |
* |
9.8 |
|
Desmodium intortum cv Greenleaf |
* |
* |
* |
* |
7.8 |
|
Dolichos Lablab K1002 |
|
* |
* |
* |
89.0 |
|
Lablab purpureus cv Rongai |
* |
* |
* |
* |
9.5 |
|
Lotononis bainesii cv Miles |
* |
|
|
|
6.1 |
|
Macrotyloma africanum CPI 24972 |
|
* |
* |
* |
6.1 |
|
Macrotyloma africanum CPI 60207 |
|
* |
* |
* |
6.1 |
|
Macrotyloma daltonii CPI 60303 |
|
* |
* |
|
16.2 |
|
Macroptilium atropurpureum cv Siratro |
* |
* |
* |
* |
9.1 |
|
Mucuna sp |
|
|
|
* |
160.5 |
|
Neonotonia wightii K75.2366 |
* |
* |
* |
* |
6.8 |
|
Rhyncosia minima CPI 52713 |
* |
* |
|
|
10.8 |
|
Rhyncosia malacophylla K18176 |
|
|
* |
* |
10.8 |
|
Stylosanthes capitata CPI 55843 |
* |
|
|
|
7.1 |
|
Stylosanthes guianensis cv Graham |
* |
* |
|
|
7.1 |
|
Stylosanthes guianensis cv Cook |
|
|
* |
* |
7.1 |
|
Stylosanthes hamata cv Verano |
* |
* |
* |
* |
7.1 |
|
Stylosanthes scabra cv Fitzroy |
* |
* |
* |
* |
7.1 |
|
Stylosanthes scabra cv Seca |
* |
|
|
|
7.1 |
|
Stylosanthes fruticosa CPI 41219A |
* |
* |
* |
* |
7.1 |
|
Vigna trilobata CPI 13671 |
* |
|
|
|
10.8 |
|
Vigna unguiculata cv M66 |
|
* |
* |
* |
82.4 |
|
Vigna unguiculata cv Red Caloona |
* |
* |
* |
|
82.4 |
Sward Experiments
The most complete data set currently available is for the first planting at the Maruba site. The pattern of dry-matter accumulation varied greatly between accessions as shown in Figure la. Single-season grain legumes such as Vigna unguiculata cv Red Caloona produced modest levels of dry matter after the first rainy season (see Harvest 1) but none thereafter. The dual purpose (forage/grain) legumes, such as Lablab purpureus (cv Rongai) were the most productive after the first and second rainy seasons (see Harvests 1 and 2) but declined thereafter (see Harvest 3). Maximum for the 18 other accessions tested was only 11% of the biomass produced by lablab purpureus. Legumes such as Alysicarpus rugosus, Stylosanthes scabra and Neonotonia wightii needed two rainy seasons to reach the production levels of an accession like Lablab purpureus. Thereafter, strong perennial species such as S. scabra and N. wightii had a clear advantage whilst annuals such as A. rugosus declined over the long dry season.
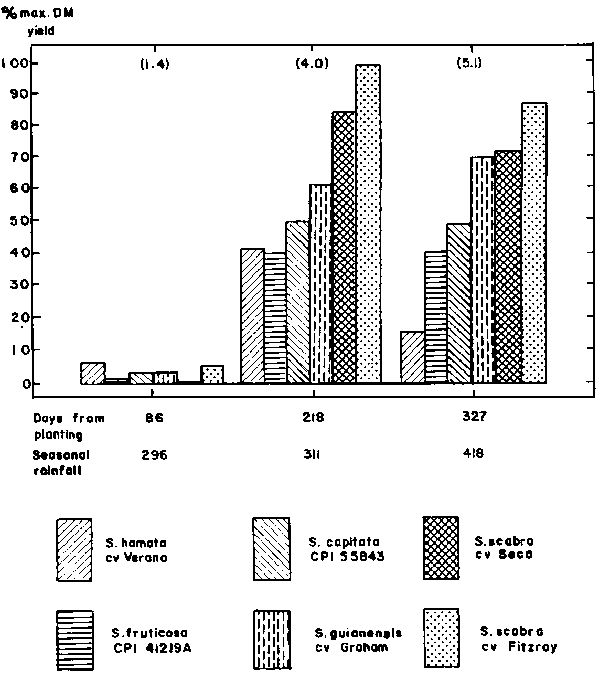
The first sward plantings at Katumani also provided an opportunity to compare the productivity of a relatively large number of Stylosanthes species under the cool dry conditions of the midlands of eastern Kenya. In all, six accessions from five species were included in this planting as shown in Figure 1b. None of the long season annuals, biennial or perennial species produced any significant levels of biomass by the end of the first season. After this time, however, the Stylos were all relatively productive with the S. scabra cultivars, Fitzroy and Seca the most outstanding (3.4 to 4.4 tonnes ha-1), followed by S. guianensis cv Graham (2.5 to 3.5 tonnes ha-1). The least adapted Stylo to this region was S. hamata cv Verano.
Figure 1b. Relative dry-matter yield (expressed as a percentage of the maximum for the particular harvest) for six Stylosanthes accessions grown at Maresha, Kenya. Other details as Figure 1a.
Leucaena Experiment
The first two destructive harvests from the Leucaena experiment have now been completed. Dry matter data is shown in Figure 2. From the preliminary data, the cultivar Peru appears to be the most productive in terms of leaf production, whilst L. diversifolia CPI 33820 was producing the largest amount of wood,
The potential role of forage and browse legumes in the semi-arid midlands of Kenya is in smallholder farming systems. Legumes could conceivably be used as inter-crops/alley-crops with cereals, in erosion control, in terrace bank stabilisation, in the improvement of degraded, non-productive grassland or as simple cut-and-carry fodder banks. The range of growth habit and persistence within the plant material being evaluated extends from the short twining annual Macrotyloma africanum to the small perennial tree Leucaena. Further experimentation (preferably on farms) is required to sort out the respective roles of material available, but the results from the experiments described here indicate that a good range of well-adapted leguminous material is now available.
Materials recommended for further testing in more favourable environments in the region (as characterised by Site 1 at Katumani NDFRS) are Cassia rotundifolia cv Wynn, Desmanthus virgatus K14456, Lablab purpureus cv Rongai, Macrotyloma africanum K14348, Neonotonia wightii K2366, Stylosanthes guianensis 'Alupe C' and Stylo scabra cv Fitzroy and 'Seca'. At less favourable sites (similar to Kiboko, Site 2) this list is reduced to Desmanthus virgatus and Stylosanthes scabra.
With the expansion of evaluation work to two other sites (3 and 4) and the inclusion of more plant material - from the Kenya Plant Quarantine Station at Muguga, from a small, indigenous plant collection and from further introduction from CSIRO, Queensland - the range of accessions being tested has recently increased. While evaluation of the following lines Aeschynomene americana cv Glenn, Cassia pilosa CPI 57503, Lotononis angolensis CPI 62202, Macrotyloma axillare cv Archer, Macrotyloma sp. (indigenous), Mucuna pruriens aff., Rhyncosia malacophylla (Synon. sennarensis), Rhyncosia totta and Stylo guianensis cv Cook is incomplete, they all show considerable promise.
The critical role of legume seed nurseries cannot be over-emphasised. Once key lines have been identified, seed requests from research stations and research or extension officers cannot be met if supply is a constraint. The nurseries should be established in areas where demands on irrigation, man-power and supervision are easily met. Work is underway this season to produce experimental quantities of seed of the promising accessions noted above.
We gratefully acknowledge the support of Mr. S.N. Muturi, Director of Agriculture, Kenya; Mr. P.K. Kusewa, Director of Katumani, NDFRS; Dr. D. Okioga, Director of the Plant Quarantine Station, KARI, Muguga and Dr. B. Woie, Director of Kiboko Range Research Station. We also thank numerous officers and staff of Katumani NDFRS. To ACIAR we are grateful for the funds provided to carry out the project.
Chabeda, A.E.O. 1976. Drought resistance in some Kenya grasses and legumes. Annual Report. National Agricultural Research Station, Kitale, Kenya.
Ibrahim, K.N. 1981. Forage plant introduction. Technical Report No. 3 Project TF-KEN 29 (NOR). FAO, Rome.
Van Soest, L.J.M. 1986. Exploration and evaluation of grasses for seed production Kenya. Eastern region of Kenya. Working paper. Project TF-KEN 29 (NOR). FAO, Rome.
Wilton, A.C. 1976. Exploration and evaluation of grasses for seed production in Kenya. Working paper. Project TF-KEN 29 (NOR). FAO, Rome.
|
|
CPI |
K |
Origin |
Site |
|
Aeschynomene americana |
91145 |
19712 |
Mexico |
1 |
|
Aeschynomene americana |
cv Glenn |
19897 |
Mexico |
1,4 |
|
Aeschynomene elegans |
37552 |
19714 |
Argentina |
1 |
|
Aeschynomene falcata |
cv Bargoo |
19715 |
Paraguay |
1,4 |
|
Aeschynomene villosa |
37235 |
19716 |
Mexico |
1 |
|
Aeschynomene villosa |
91113 |
19720 |
Mexico |
1 |
|
Alysicarpus glumaceus |
52366 |
14449 |
Madagascar |
1,2 |
|
Alysicarpus hamosus |
94491 |
14374 |
Oman |
1,2 |
|
Alysicarpus longifolius |
94490 |
14376 |
Oman |
1,2 |
|
Alysicarpus monilifer |
52343 |
14377 |
Madagascar |
1,2 |
|
Alysicarpus rugosus |
30034 |
14382 |
India |
1,2,3,4 |
|
Alysicarpus rugosus |
52348 |
14383 |
Zimbabwe |
1 |
|
Alysicarpus rugosus |
52351 |
14384 |
Malawi |
1,2,3,4 |
|
Alysicarpus rugosus |
76980 |
14447 |
Zambia |
1 |
|
Alysicarpus rugosus |
94489 |
14395 |
Ethiopia |
1,2 |
|
Alysicarpus rugosus |
- |
(50) |
Kenya |
1,2 |
|
Alysicarpus vaginalis |
34149 |
14386 |
Nicaragua |
1,2,4 |
|
Arachis monticola |
CQ990 |
14388 |
- |
1 |
|
Arachis pintoi |
58113 |
14416 |
Brazil |
1 |
|
Cassia pilosa |
57503 |
14451 |
Venezuela |
1,2,4 |
|
Cassia rotundifolia |
86172 |
19724 |
Mexico |
1 |
|
Cassia rotundifolia |
86178 |
19725 |
Mexico |
1 |
|
Cassia rotundifolia |
Q10057 |
14450 |
Brazil |
1 |
|
Cassia rotundifolia |
cv. Wynn |
18177 |
- |
1,2,3,4 |
|
Centrosema acutifolium |
92874 |
14391 |
Brazil |
1,2 |
|
Centrosema acutifolium |
94303 |
14390 |
Colombia |
1 |
|
Centrosema brasilianus |
55698 |
14417 |
Brazil |
1,2,4 |
|
Centrosema macrocarpum aff |
78358 |
20170 |
Brazil |
4 |
|
Centrosema pascuorum |
65950 |
14392 |
Equador |
1,2,3,4 |
|
Centrosema pascuorum |
cv Cavalcade |
14418 |
- |
1,2,3,4 |
|
Centrosema plumieri |
60477 |
14393 |
Brazil |
1,2 |
|
Centrosema plumieri |
82269 |
14394 |
Cuba |
1,2 |
|
Centrosema pubescens |
43197 |
20171 |
Colombia |
4 |
|
Centrosema pubescens |
46543 |
14395 |
Guatemala |
1,2 |
|
Centrosema pubescens |
58575 |
20172 |
Colombia |
4 |
|
Centrosema pubescens |
63895 |
14396 |
Brazil |
1,2,4 |
|
Centrosema pubescens |
79630 |
20173 |
- |
4 |
|
Centrosema pubescens |
92721 |
14397 |
Colombia |
1 |
|
Centrosema pubescens |
cv Belalto |
14332 |
- |
1,2 |
|
Centrosema pubescens |
cv Centro |
14419 |
- |
1,2 |
|
Centrosema schottii |
76010 |
14398 |
Mexico |
1,2,3 |
|
Centrosema schottii |
82271 |
14445 |
Cuba |
1,2,3 |
|
Centrosema virginianum |
CQ2748 |
14399 |
- |
1,2,3,4 |
|
Centrosema virginianum |
91142 |
14400 |
Mexico |
1,2,3,4 |
|
Clitoria ternatea |
47187 |
14402 |
Sudan |
1,2,3,4 |
|
Clitoria ternatea |
48337 |
14403 |
Tanzania |
1,2,3,4 |
|
Clitoria ternatea |
49963 |
14404 |
- |
1,2,3,4 |
|
Desmanthus bicocnutus |
91162 |
14438 |
Mexico |
1 |
|
Desmanthus subulatus |
90857 |
14406 |
Mexico |
1 |
|
Desmanthus virgatus |
40071 |
14456 |
Brazil |
1,2,3,4 |
|
Desmanthus virgatus |
55719 |
14407 |
Venezuela |
1,2 |
|
Desmanthus virgatus |
65947 |
14405 |
Equador |
1,2 |
|
Desmanthus virgatus |
78373 |
14408 |
Argentina |
1,2 |
|
Desmanthus virgatus |
83570 |
14409 |
Brazil |
1,2,4 |
|
Desmanthus virgatus |
84508 |
14410 |
Mexico |
1,2 |
|
Desmanthus virgatus |
85178 |
14411 |
Mexico |
1,2 |
|
Desmanthus virgatus |
90750 |
14412 |
Mexico |
1,2 |
|
Desmanthus virgatus |
91146 |
14413 |
Mexico |
1,2 |
|
Desmanthus virgatus |
91326 |
14414 |
Mexico |
1,2,4 |
|
Desmanthus virgatus |
92818 |
14415 |
Belize |
1,2,4 |
|
Desmodium dichotosum |
47186 |
- |
- |
1,2 |
|
Desmodium intortum |
cv Greenleaf |
14455 |
- |
1,2,3,4 |
|
Desmodium intortum |
91135 |
14338 |
Mexico |
1,2 |
|
Desmodium oringlei |
37232 |
14334 |
Mexico |
1,2 |
|
Desmodium prostratum aff |
91232 |
14336 |
Mexico |
1,2 |
|
Desmodium setigeruam |
52431 |
14340 |
Malawi |
1,2 |
|
Desmodium subsericeum |
78402 |
14335 |
Argentina |
1,2 |
|
Desmodium wigginsii |
90418 |
14337 |
U.S.A. |
1,2 |
|
Desmodium sp. D |
91186 |
14453 |
Mexico |
1 |
|
Desmodium sp. D |
91212 |
14333 |
Mexico |
1,2 |
|
Dolichos sp. |
24973 |
14452 |
Zimbabwe |
1,2,4 |
|
Dolichos sericeus aff |
- |
(128) |
Kenya |
1,4 |
|
Lablab purpureus |
30702 |
14466 |
Burma |
1 |
|
Lablab purpureus |
41222 |
14341 |
Burma |
1,2 |
|
Lablab purpureus |
cv Highworth |
14463 |
- |
1,2 |
|
Lablab purpureus |
cv Rongai |
14420 |
Kenya |
1,2,3,4 |
|
Lablab purpureus |
- |
1002 |
Kenya |
1,2,3,4 |
|
Lespedeza striate |
cv Kaloe |
19899 |
U.S.A. |
1,4 |
|
Lotononis angolensis |
62202 |
14435 |
- |
1,2,4 |
|
Lotononis bainesii |
cv Miles |
14421 |
- |
1,2,4 |
|
Macroptilium atropurpureum |
84989 |
14343 |
Mexico |
1,4 |
|
Macroptilium atropurpureum |
84999 |
14344 |
Mexico |
1,2 |
|
Macroptilium atropurpureum |
90748 |
14468 |
Mexico |
1,4 |
|
Macroptilium atropurpureum |
90776 |
14469 |
Mexico |
1,4 |
|
Macroptilium atropurpureum |
90821 |
14465 |
Mexico |
1 |
|
Macroptilium atropurpureum |
cv Siratro |
14461 |
Mexico |
1,2,3,4 |
|
Macroptilium heterophyllum |
90448 |
14345 |
Mexico |
1,4 |
|
Macroptilium heterophyllum |
91144 |
14467 |
Mexico |
1 |
|
Macroptilium heterophyllum |
91222 |
14346 |
Mexico |
1 |
|
Macroptilium lathyroides |
cv Murray |
14464 |
India |
1,2 |
|
Macroptilium longipendunculatum |
55751 |
14422 |
Brazil |
1,2 |
|
Macroptilium martii |
49780 |
14347 |
Brazil |
1,2,4 |
|
Macroptilium prostrate |
78450 |
14342 |
Argentina |
1 |
|
Macrotyloma africanum |
24972 |
14348 |
Zambia |
1,2,3,4 |
|
Macrotyloma africanum |
60207 |
14349 |
Zambia |
1,2,3,4 |
|
Macrotyloma axillare |
cv Archer |
14462 |
- |
1,4 |
|
Macrotyloma daltonii |
60303 |
14350 |
Namibia |
1,2,3,4 |
|
Macrotyloma daltonii |
94496 |
14351 |
- |
1 |
|
Macrotyloma uniflorum |
cv Leichardt |
14460 |
- |
1,2 |
|
Macrotyloma sp. |
- |
(129) |
Kenya |
1,4 |
|
Medicago rugosa |
cv Paraponto |
19905 |
- |
1,4 |
|
Medicago rugosa |
cv Sapo Gama |
19906 |
- |
1,4 |
|
Medicago sativa |
cv H. River |
19900 |
- |
1,4 |
|
Medicago scutella |
cv Sava |
19907 |
- |
1,4 |
|
Medicago trunculata |
cv Jemalong |
19908 |
Australia |
1,4 |
|
Mucuna pruriens aff |
- |
(119) |
Kenya |
1,4 |
|
Neonotonia wightii |
- |
2366 (67) |
Kenya |
1,2,3,4 |
|
Neonotonia wightii |
- |
(35) |
Kenya |
1,2,3,4 |
|
Neonotonia wightii |
- |
(150) |
Kenya |
1,4 |
|
Rhyncosia densiflora |
52690 |
14353 |
Tanzania |
1,2 |
|
Rhyncosia edulis |
52127 |
14352 |
Paraguay |
1,2 |
|
Rhyncosia malacophylla |
- |
18176 |
Tanzania |
1,2,3,4 |
|
Rhyncosia minima |
52713 |
14355 |
Tanzania |
1,2,3 |
|
Rhyncosia minima |
78473 |
14356 |
Argentina |
1 |
|
Rhyncosia minima |
84953 |
14357 |
Mexico |
1,2 |
|
Rhyncosia minima aff |
- |
(14) |
Kenya |
1,2 |
|
Rhyncosia minima aff |
- |
(41) |
Kenya |
1,2 |
|
Rhyncosia minima aff |
- |
(45) |
Kenya |
1,2 |
|
Rhyncosia totta |
52742 |
14358 |
Zambia |
1,4 |
|
Stylosanthes capitata |
55843 |
14459 |
Brazil |
1,2,4 |
|
Stylosanthes fruticosa |
412194 |
14426 |
Sudan |
1,2,3,4 |
|
Stylosanthes fruticosa |
- |
(33) |
Kenya |
1,2 |
|
Stylosanthes guianensis |
79637 |
14364 |
Brazil |
1,2,4 |
|
Stylosanthes guianensis |
- |
Alupe C |
- |
1,2,4 |
|
Stylosanthes guianensis |
- |
Alupe I |
- |
1,2 |
|
Stylosanthes guianensis |
cv Cook |
18189 |
Colombia |
1,2,3,4 |
|
Stylosanthes guianensis |
cv Graham |
14427 |
Bolivia |
1,2,3,4 |
|
Stylosanthes guianensis |
cv Oxley |
19901 |
Argentina |
1,4 |
|
Stylosanthes hamata |
49080 |
14365 |
Colombia |
1,2 |
|
Stylosanthes hamata |
70522 |
14366 |
U.S.A. |
1,2 |
|
Stylosanthes hamata |
73507 |
14367 |
Antigua |
1,2 |
|
Stylosanthes hamata |
cv Verano |
14428 |
Venezuela |
1,2,3,4 |
|
Stylosanthes humilis |
61674 |
14368 |
Venezuela |
1,2 |
|
Stylosanthes humilis |
cv Greenvale |
14429 |
- |
1,2 |
|
Stylosanthes scabra |
55856 |
18190 |
Brazil |
1,2 |
|
Stylosanthes scabra |
cv Fitzroy |
14431 |
- |
1,2,3,4 |
|
Stylosanthes scabra |
cv Seca |
14430 |
- |
1,2,3,4 |
|
Stylosanthes scabra |
- |
8115 |
- |
1,2 |
|
Stylosanthes scabra |
- |
8111 |
- |
1,2 |
|
Stylosanthes scabra |
- |
82105 |
- |
1,2 |
|
Stylosanthes subsericea |
38605 |
14457 |
Guatemala |
1,2 |
|
Stylosanthes sympodialis |
67704(B) |
14369 |
Equador |
1,2 |
|
Stylosanthes viscosa |
34904 |
14371 |
Brazil |
1,2 |
|
Stylosanthes sp. |
85895 |
14359 |
Mexico |
1,2 |
|
Stylosanthes sp. |
85899 |
14360 |
Mexico |
1,2 |
|
Stylosanthes sp. |
86137 |
14361 |
Mexico |
1,2 |
|
Stylosanthes sp. |
87469 |
14458 |
Mexico |
1,2 |
|
Stylosanthes sp. |
87479 |
14362 |
Mexico |
1,2 |
|
Stylosanthes sp. |
87484 |
14423 |
Mexico |
1,2 |
|
Stylosanthes sp. |
87485 |
14424 |
Mexico |
1,2 |
|
Stylosanthes sp. |
87487 |
14425 |
Mexico |
1,2 |
|
Stylosanthes sp. |
91138 |
14363 |
Mexico |
1,2 |
|
Trifolium repens |
cv Haifa |
19902 |
Israel |
1,4 |
|
Trifolium sp. |
- |
(26) |
Kenya |
1,3 |
|
Vigna ambacensis |
47188 |
14433 |
Sudan |
1,2 |
|
Vigna frutescens |
- |
(126) |
Kenya |
1 |
|
Vigna luteola |
ILCA 113 |
18172 |
Belize |
1,4 |
|
Vigna oblongifolia |
60430 |
14372 |
- |
1,2 |
|
Vigna praecox |
- |
(130) |
Kenya |
4 |
|
Vigna trilobata |
13671 |
14434 |
- |
1,2,3 |
|
Vigna unguiculata |
60442 |
14313 |
- |
1,2 |
|
Vigna unguiculata |
60452 |
|
Kenya |
1,2,4 |
|
Vigna unguiculata |
cv Red Caloona |
14436 |
- |
1,2,3,4 |
|
Vigna unguiculata |
- |
M66 |
Kenya |
1,2,3,4 |
|
Vigna vexillata |
- |
(122) |
Kenya |
1,4 |
|
Voandzeia subterranea |
- |
18174 |
Mali |
4 |
|
Zornia sp. |
- |
(123) |
Kenya |
1 |