C.B. Katongole1) & S. Gombe2)
1. Department of Veterinary Physiological Sciences, Makerere University, P.O. Box 7062, Kampala, Uganda.2. Department of Animal Physiology, University of Nairobi, P.O. Box 30197, Nairobi, Kenya.
Introduction
Material and methods
Results
Discussion
Acknowledgements
Summary
Plasma progesterone and oestradiol in females and plasma testosterone in males were determined by specific radioimmunoassays (RIA) in Small East African goats. During the oestrous cycles plasma progesterone was found to vary between 2 and 18 nM/l, progesterone was high during pregnancy reaching a maximum of 21.10 ± 1.46 nM/l during the last month of pregnancy, but was below 3 nM/l for several months during the postpartum period. Oestradiol-17B levels varied from 120 to 900 pM/l during a cycle and were at 554 ± 424 pM/l during the second half of pregnancy. During the postpartum period oestrogens were as high as in the second half of pregnancy. Most animals became pregnant in March coinciding with lush pastures after the start of the long rains. Hormone levels postpartum suggest absence of ovulation and corpora lutea formation for several months, most probably due to LH deficiency, resulting in a longer kidding interval. Testosterone levels in male goats varied between 0.5 - 12 nM/l indicative of episodic release of LH unrelated to climatic conditions.
Sheep and goats are seasonal breeders in temperate climates, with the breeding season becoming longer as the equator is approached (Hafez, 1952). Tropical breeds of goats are thought to be aseasonally polyoestrus and thus can breed the year round (Mason & Maule, 1960; de Hass & Horst, 1979).
In seasonal breeders there are a number of factors which either stimulate or suppress the breeding activity. In sheep a high plane of nutrition such as caused by "flushing" is known to influence the rate of ovulation and increases the numbers of twins and triplets. The nutritional value of tropical pastures fluctuates widely between the dry and the rainy seasons. It was therefore considered necessary to measure reproductive hormones in goats at different times of the year to determine seasonal effects. This information is required before managerial decisions or artificial manipulations of the reproductive process can be undertaken in order to increase reproductive and productive efficiencies.
Mature does and bucks of the Small East African (SEA) goat were bought in 1981 and kept at Makerere University campus. Management of the animals and regular detection of oestrus in the flock have been reported elsewhere (Katongole, 1983). Au animals were bled regularly, once a week for young animals and twice a week for adult animals for a period of 16 months. Blood plasma was kept at -20°C and transferred in packed fee to Nairobi for assay.
Plasma progesterone, oestradiol and testosterone were assayed in accordance with the WHO protocol (WHO RIA Methods Manual). In each case 200 to 500 m l of plasma was mixed with 1000 c.p.m. tritiated hormone to estimate procedural loss. The sample was then extracted with ten volumes of diethylether and after freezing the aqueous phase the extracts were poured off into glass tubes. The ether extracts were dried under nitrogen and reconstituted in 2 ml of PBS-Gel. Two aliquots of 500 m l were taken for specific radioimmunoassay (RIA) and one aliquot of 500 m l for recovery estimation. To each sample or standard tube was added 10 000 c.p.m. in 100 m l of PBS-Gel of the appropriate tracer hormone and 100 m l of the specific antibody dilution. The mixture was incubated at 4°C for 18-24 hours before separating bound from free hormone using dextran-coated charcoal. After adding 200 m l of the charcoal mixture the whole was vortexed and stood at 4°C for 15-20 minutes before centrifuging at 4°C for five minutes at 500 g. The bound hormone in the supernatant was counted in 4 ml of PPO-toluene scintillation fluid. For the standard curve the logit of B/Bo was plotted against log dose from which the samples were read. The concentration of the hormone was then corrected for procedural losses and dilution and expressed as nM/l for progesterone and testosterone and as pM/l for oestradiol.
The hormone assays were pooled on a monthly basis. Figure 1 gives progesterone and oestradiol results in four adult does which were showing psychic oestrus and which subsequently became pregnant and produced normal kids.
PROGESTERONE AND OESTRADIOL LEVELS DURING THE OESTROUS CYCLE
Animals showing oestrous periods before pregnancy had progesterone levels varying between 2.0 and 9.2 nM/l and oestradiol levels of 120 to 720 pM/l. Whereas low progesterone levels are most likely to have been around oestrus during the follicular phase, the higher levels occurred during the luteal phase.
PROGESTERONE AND OESTRADIOL LEVELS DURING PREGNANCY
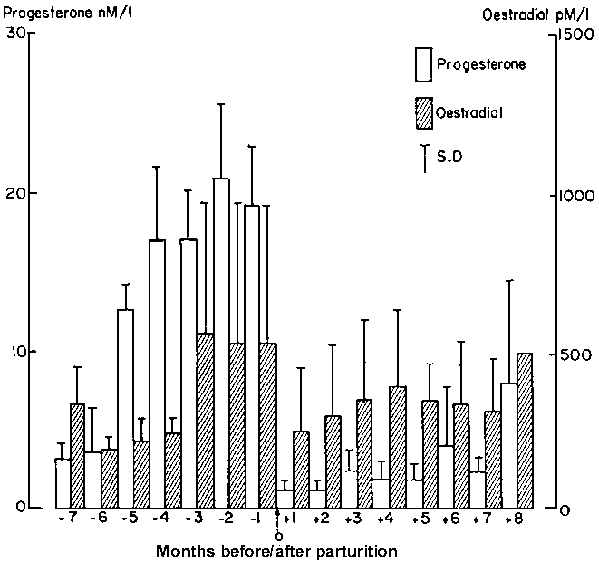
Figure 2 shows hormone levels of several animals during pregnancy and the postpartum period at about the time of parturition. Reference should also be made to Figure 1 for the individual animals.
During the first month of pregnancy progesterone was found to be similar to the maximal levels of cycling animals at 9.3 ± 1.51 nM/l (n = 6) but had risen to 17.1 ± 1.98 nM/l by the second month of pregnancy and maximal levels of 21.1 ± 1.46 nM/l were present in the last month of pregnancy. In some animals progesterone levels as high as 27.3 nM/l were recorded. Oestradiol levels rose from 205.8 ± 30.1 pM/l during the first month of gestation to 554 ± 424 pM/l by the third month of pregnancy and remained at that level until parturition. The highest levels of oestrogens of over 1000 pM/l were recorded in some of the animals during the last month of pregnancy.
Figure 1. Plasma progesterone and oestradiol levels in four SEA goats
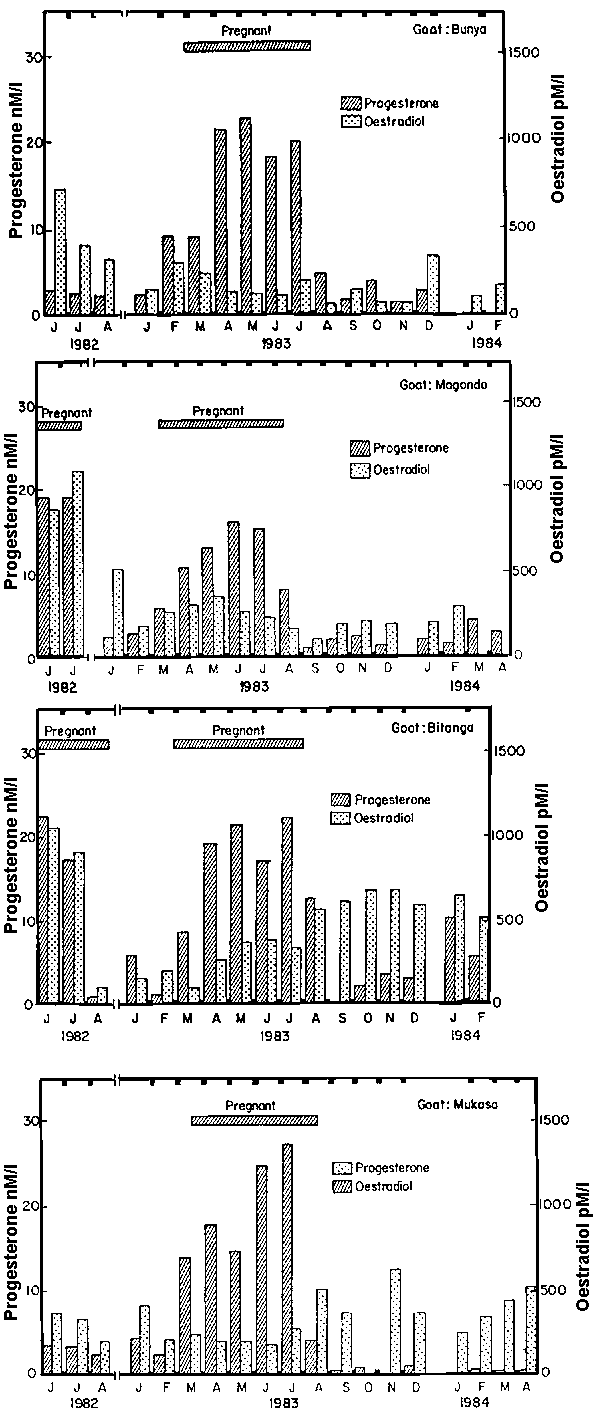
Figure 2. Plasma progesterone and oestradiol levels during pregnancy and the postpartum period ( ± SD, n = 6)
± SD, n = 6)
POSTPARTUM PROGESTERONE AND OESTRADIOL LEVELS
Hormone levels during the postpartum period are shown in Figures 1 and 2. In the majority of animals the progesterone levels recorded were lowest in the first month postpartum, were below 3 nM/l for five or more months and in some exceptional cases remained low for up to eight months. Oestradiol levels ranged from 300 to 600 pM/l which were as high as the levels found in the second half of pregnancy. It would appear that during the postpartum period oestradiol was the dominant hormone for some time.
PREPUBERTAL AND PUBERTAL PROGESTERONE AND OESTRADIOL LEVELS
Figure 3 shows hormone levels recorded in two animals in samples taken before and after puberty. In one, first mating was not recorded until 15 months of age at a weight of 16.5 kg. In another first mating was recorded at eight months at a weight of 16.0 kg. Before puberty, progesterone level did not exceed 2 nM/l whereas after puberty progesterone ranged from 3 to 17 nM/l.
Oestradiol ranged from 300 to 900 pM/l being the dominant hormone before puberty whereas after puberty the range was 50 to 500 pM/l.
PLASMA TESTOSTERONE IN ADULT MALE GOATS
Figure 4 shows plasma testosterone in two adult male goats. This was found to vary greatly between 0.5 and 12.0 nM/l.
The levels of progesterone and oestradiol reported in this paper show great variation. As expected the levels vary according to the stage of the reproductive cycle or presence of pregnancy. In goats during the follicular phase around oestrus Thornburn & Schneider (1972) reported low progesterone levels of less than 0.2 ng/ml (ca 0.64 nM/l) and maximal levels were found by day 10 of the cycle. On the other hand oestrogens have been found to peak at or around oestrus (Scaramuzzi, Caldwell & Moor, 1970).
In the present study progesterone levels above 3.0 nM/l were taken to be indicative of normal cycling with ovulation and corpus luteum formation if the animals were not pregnant. The levels of progesterone reported here of 2-18 nM/l in cycling animals are comparable to those reported by Kakusya (1979) in pygmy goats although his maximal levels are much higher (9.3 ± 0.3 ng/ml (ca 29.5 nM/l) with a range of 7.2 - 12.2 ng/ml (ca 22.9 - 38.8 nM/l)). Since the present study reports monthly figures the lower maximal figures could be due to mixing high level samples with low hormone level samples.
Figure 4. Plasma testosterone in two adult bucks
Oestrogen levels of 120 - 900 pM/l during the cycle are in the range reported by other authors for the goat (Thornburn & Schneider, 1972; Kakusya, 1979). It has not been possible to show any seasonal effect in the level of circulating hormones in the present study, although there was a peak in conceptions in March. This could be due to a "flushing" effect at the beginning of the long rains following a prolonged dry period in December to February.
Progesterone was maximal during pregnancy, particularly in the second half. Oestrogens were also higher during the latter part of pregnancy. The trends in hormone levels are similar to those reported by other authors for the goat (Thornburn & Schneider, 1972; Kakusya, 1979; Mgongo, Gombe & Ogaa, 1983). In the goat pregnancy maintenance has been shown to be dependent solely on progesterone from the corpus luteum (Bustle, 1978) and there is a rise in progesterone production after 60 days of pregnancy due to placental lactogen stimulation which "rejuvenates" the corpus luteum to full function (Thornburn, Charllis & Currie, 1977).
Low progesterone levels postpartum are an indication of lack of ovulation and corpus luteum formation and thus the animals could not become pregnant for several months. This could be an explanation for the long kidding interval of 296.7 ± 8.5 days reported in the indigenous goat (Sacker & Trail, 1966). Another significant finding is the rather high levels of oestrogens in some animals postpartum, in some cases as high as those found in late pregnancy. This could be indicative of the existence of cystic ovaries and a deficiency of LH resulting in failure of ovulation. It is known for sheep that during pregnancy there is a marked reduction in the pituitary contents of LH reaching a nadir at parturition at levels below 25 per cent of the amount needed to induce ovulation (Moss et al, 1980). During the postpartum period there is recovery, pituitary LH returning to normal by about day 35 (Moss et al, 1980; Crowder et al, 1982). It has also been shown that high concentrations of oestradiol similar to those present in the last week or two of gestation reduce the pituitary content of LH to very low levels. The high concentration of oestradiol also causes a dramatic (98 per cent) reduction of mRNA encoded for the beta submit of LH in the anterior pituitary of ewes (Glass, Aman & Nett, 1983; Nilson et al, 1983). The results from the present study would tend to indicate that there is a prolonged postpartum anoestrus/lack of ovulation because of high circulating oestrogens with a concomitant lack of adequate release of LH. Since most indigenous goats suckle their kids for prolonged periods, this may also be a factor in prolonging the postpartum anoestrus/annovulation as has been shown in other species (Wiltbank & Cook, 1958; Short et al, 1972). Poor or inadequate nutrition is also likely to occur during the dry periods and that too would exacerbate the postpartum anoestrus/annovulation (Wiltbank et al, 1962; Corah, Dunn & Kaltenbach, 1975; Bellows & Short 1978). On the other hand a higher plane of nutrition and shortened lactation will reduce the postpartum anoestrus and shorten the kidding interval. Lastly, heat stress could occur during the dry hot weather and this is known to cause reproductive failure in both males and females (Devendra & Burns, 1983).
In adult males plasma testosterone showed wide levels of variation with no obvious pattern. This may be due to episodic release of LH from the pituitary as occurs in the ram and the bull (Katongole, Naftolin & Short, 1971; 1974).
The authors are grateful for financial assistance from the International Foundation for Science and Makerere University. J. Osaso, F. Kariba and D. Ndegwa of the University of Nairobi assisted with the hormonal assays. J. Nkugwa, A. Iga and the late J. Mukasa assisted with the animal work. Miss L.R.N. Semuwemba typed the draft manuscript.
Small East African buck in house compound in Uganda