3.1 Dehydration
3.2 Channels of water loss
3.3 Channels of water gain
In all animals, the body water pool must remain reasonably constant in the long term, although livestock adapted to arid areas may be able to tolerate fairly large short-term fluctuations. As already suggested, animals drink primarily to replace lost fluid, rather than in anticipation of future needs. Thus water loss largely dictates water gain, and will therefore be dealt with first. The size and speed of body fluid loss is affected partly by the factors outlined in chapter 4 and partly by the tolerance of the particular species or age group to dehydration.
The symptoms of dehydration as described in man probably apply also to animals, although at greater levels of body water depletion. In man, signs of thirst are very strong when as little as 2% of bodyweight has been lost, but do not get progressively worse as dehydration proceeds; after 4% weight loss the mouth is very dry and at 8% the tongue is swollen and speech difficult. Severe thirst and some mental derangement are apparent at 10%; at 12%, recovery is only possible with some assistance and it may be necessary to give fluids by injection or per rectum. The actual lethal limit for man is probably between 15 and 25% of bodyweight (Mount, 1979).
By contrast, a 10% decrease in fat-free bodyweight (i.e. a 9% weight loss in a tropical ungulate) represents only moderate dehydration. It is countered by withdrawing fluid from the alimentary tract, from tissue spaces and then from the cells to maintain circulating blood volume, provided that the osmotic pressure of the blood is sufficiently high. Essential parts of the body, including the central nervous system, heart and skeleton, contribute little of this water: the major loss occurs from connective tissues, muscle and skin. Consequently the first and most important clinical sign of dehydration is dryness and wrinkling of the skin which subsides slowly after being picked up into a fold. It gives the body and face a shrunken appearance. The eyeballs recede into the sockets, but this is due to pert-orbital fat loss. According to Blood and Henderson (1960), there is an increase in tissue metabolism of fat, then carbohydrate and finally protein to produce metabolic water, but Schmidt-Nielsen (1975) pointed out that much of the water produced in this way could be used up dissipating the heat associated with its production. Loss of bodyweight occurs rapidly, along with muscular weakness, lack of appetite and increased thirst. The increased tissue metabolism under relatively anaerobic conditions results in the formation of acid metabolites, acidosis, and therefore a rise in the respiratory rate. The temperature rises slightly, the heart rate increases and the pulse has a small amplitude and low pressure (Blood and Henderson, 1960).
In a hot environment, the signs of more severe dehydration (15 to 30% decrease in fat-free bodyweight) are acute. The immediate source of water loss is from the bloodstream. If the water is not replenished, the blood becomes more viscous, placing an additional load on the heart. The heart responds by an increase in stroke rate but a decrease in stroke volume, causing a decrease in blood circulation. As a result the body temperature rises, because one of the most important functions of the bloodstream is heat transfer from the body core to the skin. If this condition is not reversed by spraying or immersion in cold water plus rehydration, death will occur as a result of an explosive rise in body temperature, rather than as a direct effect of water depletion. Clinical signs of hyperthermia occur in many animals at a rectal temperature of about 39.5°C. Heart and respiratory rates increase, with a weak, large-amplitude pulse, and nervous system activity is depressed, so that the animal becomes dull, stumbles while walking and tends to lie down. When the temperature reaches 42.0°C, respiration is laboured arid general distress is evident. Beyond this point the pulse becomes very rapid and weak, and respiration shallow and irregular. The prime cause of death is probably from depression of the respiratory centre in the brain. Collapse, convulsions and terminal coma occur-in most species when a temperature of about 42.0°C is reached (Adolph and Dill, 1938; Blood and Henderson, 1960; Schmidt-Nielsen, 1975).
The most common cause of dehydration in a hot environment is loss of water due to evaporative cooling. Water turnover rates have not been measured in dehydrated animals in sub-Saharan Africa, but a study was carried out with camels, cattle and sheep in the Australian desert, where the maximum ambient temperature was 42°C and solar radiation was measured at 1160 for W.m-2. The cattle (Bos taurus) lost 7-8% of their body water per day and survived for 3-4 days, Merino sheep lost 4-6% per day and lived for 6-8 days and camels lost 1-2% and survived for 15-20 days (Macfarlane and Howard, 1972).
In a cooler environment, death from dehydration occurs more slowly, with a deterioration of body functions occurring at a greater degree of dehydration. Much of this deterioration can probably be related to the breakdown of the normal functions of the blood. The most important of these functions include: transport of nutrients, metabolites, excretory products, gases, hormones, and non-respiratory blood cells, transfer of heat, and transmission of force for ultrafiltration in capillaries and kidneys (Schmidt-Nielsen, 1975).
The commonest cause of dehydration in cool environments is diarrhoea, which is often combined with systemic states such as toxaemia or septicaemia. Any form of gastro-intestinal impaction, obstruction or distension also produces dehydration by stimulating saliva and gut secretions (Blood and Henderson, 1960). In sub-Saharan Africa, scours and heat stress frequently occur together and are probably the main cause of death in young stock.
The ability to withstand dehydration has been ascribed to the maintenance of a normal plasma volume. This volume is maintained primarily by the osmotic pressure of the plasma proteins. These proteins leak out of the vascular bed much more quickly in animals such as cattle (Bos taurus), which are not arid-adapted, than in camels, which are good at maintaining their plasma volume (Siebert, 1973). In man, the most important event in heat acclimatization is the expansion of the plasma volume (Senay et al, 1976). Lactating Bedouin goats are reported to have an expanded plasma volume of 8.5% bodyweight, which means that there can be a 40% reduction before the plasma volume equates with the 5% value found in most other ruminants (Shkolnik et al, 1979). Similar sized reductions in plasma volume have been recorded in Indian desert sheep, but it is not clear whether the initial plasma volume was an abnormally large proportion of bodyweight (Purohit et al, 1972). Although the plasma volume decreased, the plasma sodium concentration remained constant, associated with a doubling of urinary sodium levels (Gosh et al, 1976).
Under African ranching conditions, livestock use 5 to 30% of their total body water pool per day (King, 1979). This loss is reduced to as little as 1.5% in dehydrated, arid-adapted animals such as the camel (Schmidt-Nielsen, 1965).
There are four main avenues of water loss: evaporation, urine, faeces and lactation.
Evaporative cooling may account for 20 - 30% of the total dissipation of the effective radiative heat load in a tropical ruminant and 80% of the water loss (Taylor, 1972; Finch, 1972b). In addition there is an obligatory water loss from the respiratory tract which is probably >10%, and a small insensible water loss through the skin (King and Finch, 1982). In general biological terms, the smaller the animal the larger the surface area to volume ratio and thus the greater the efficiency of evaporative cooling. On the other hand, the larger the animal the greater the volume per unit surface area and hence the larger the water reserve which can be used for evaporative cooling (Edney, 1966).
The relative contribution of sweating or panting to evaporative heat dissipation in a number of different domestic animals is shown in Table 8. Among ungulates in Africa, buffaloes, camels, cattle, donkeys and some of the larger antelopes sweat, whereas wildebeest, oryx, sheep, goats and many smaller gazelles pant. Insensible perspiration and non-panting respiratory heat loss account for a relatively small amount of the total loss in hot, dry conditions. Although panting seems to be completely adequate as the sole means of heat loss among sheep, sweating can also be important in closely shorn or haired sheep over the first few hours of heat stress (Hofmeyr et al, 1969; Taylor et al, 1969; Jenkinson, 1972).
Table 8. The relative contributions (%) of sweating and panting to evaporative heat loss in various domestic animals in a hot, dry environment.
|
|
Relative contribution (%) to evaporative heat loss |
|||||
|
Donkey |
Camel |
Cow |
Sheep/goat |
Dog |
Pig |
|
|
Sweat |
100 |
95 |
65 |
40 |
10 |
0 |
|
Pant |
0 |
5 |
35 |
60 |
90 |
100 |
Source: Jenkinson (1972).
There are a number of reasons why smaller animals, with their relatively higher heat production and absorption, should pant rather than sweat. Panting appears to be the more efficient of the two methods of evaporative cooling. Both methods use latent heat from the body core, but sweating can also use solar radiation on the body surface. Panting also provides its own airflow over moist surfaces, thus facilitating evaporation. Salt and electrolytes are not lost, as in sweating, unless the saliva drips out of the mouth. Finally, panting cools the nasal and oral passages whence cool blood flows into the venous sinus, bathing the carotid plexus. Thus the blood supply to the brain can be kept cool, even when the body temperature is rising (Taylor and Lyman, 1972). The disadvantages of panting include a risk of respiratory alkalosis, particularly in the goat (Jenkinson, 1972), and the increase in work and therefore heat production by the respiratory muscles. However, much of this work is reduced by the elastic property of the respiratory system, which has its own natural frequency of oscillation. The high respiratory rate associated with panting has the effect of keeping the system oscillating at its own resonant frequency with the minimum of muscular effort. Thus, the thermoregulatory efficiency of panting is high in such species as sheep, which show no increase in total body heat production above normal levels (Hales and Brown, 1974).
As 1 g of water changes from liquid to vapour, whether by panting or sweating, it binds about 2425 J of heat. In terms of heat exchange this is a very efficient use of water when it is realized that to heat 1 g of water from freezing to boiling point requires only 490 J. Nevertheless, evaporation can represent a very significant loss of body water. A fully hydrated camel weighing 260 kg lost 91 of water a day through sweat when standing in the desert sun. This quantity represented a loss of 4% of total bodyweight, and a loss much in excess of 25% would probably be fatal. Assuming that heat load and therefore evaporation are proportional to body surface, then water loss under hot, desert conditions increases exponentially with decreasing size. There is very little difference in water loss per hour in the camel at 1.0%, and man at 1.5%, but the rate in animals weighing 2.5 kg is nearly 5%. Many animals also have lower lethal limits than the camel (Schmidt-Nielsen, 1965). The need to preserve vital functions, as an animal becomes dehydrated, results in a reduction in the rate of evaporative cooling. The sequel to this reduction is either a rise in body temperature or a depression of heat production.
Pathological conditions of the respiratory tract and skin may interfere with normal evaporation. For example, dermatitis from mange mites, virus pox or bacteria may result in excessive fluid exudate and require parenteral administration of fluids (Blood and Henderson, 1960).
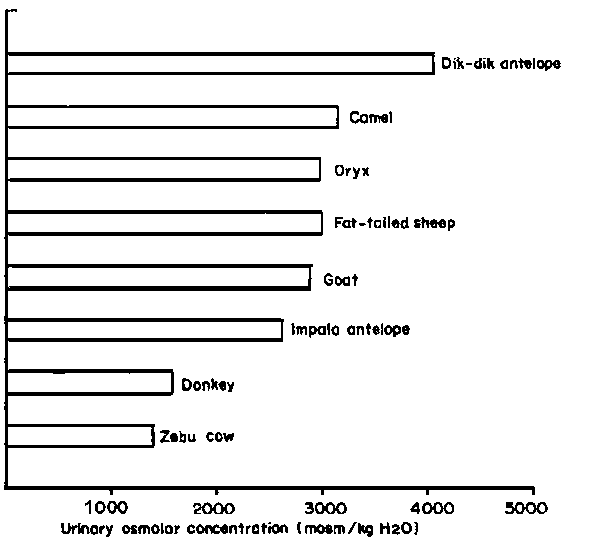
Livestock species vary in terms of their ability to concentrate urine and/or decrease renal urine flow and retain metabolites in the body fluids. Camels, sheep and goats are better adapted to arid conditions in this sense than zebu cattle or donkeys, as shown in Figure 3 (Maloiy and Taylor, 1971; Maloiy, 1972). Although less water tends to be lost from the body through urine than through the faeces, the scope for varying urine concentration and flow is greater. Thus, the desert sheep is only 30% more efficient than the zebu at extracting moisture from its faeces but at least 100% more efficient at concentrating its urine.
Among African ungulates, the highest levels of urine concentration have been recorded for the gerenuk and dik-dik at over 4000 mosm.kg-1 (Hoppe, 1976) - a low figure compared with desert rodents, which can concentrate their urine to over 9000 mosm.kg-1 (MacMillen, 1972). The reason for the difference may be that large animals cannot avoid high environmental heat loads, and therefore have high water losses from evaporation. The amount of water saved by concentrating their urine is relatively little compared with that lost by evaporation. In contrast, the entire water balance of small rodents living in burrows can be designed round an efficient renal mechanism for water conservation (Schmidt-Nielsen, 1972).
Figure 3. The maximal urinary osmolar concentrations of several East African mammals under severe dehydration. (Source: Maloiy (1972)
Diseases of the urinary system will upset the normal water balance of the animal but are masked by symptoms of pain and toxaemia. The commonest pathological conditions are cystitis and pyelonephritis in cows following a difficult parturition, and urolithiasis in castrated males (Blood and Henderson, 1960).
Faecal water is a potentially larger source of water loss than urine. This loss is not confined to exogenous water, because half the total body water pool can pass through the salivary glands and rumen per day. Therefore the ability to extract and reabsorb faecal water in the colon is important. A breakdown of the water reabsorption mechanisms in the large intestine results in diarrhoea and can cause dehydration. Bos taurus cattle can reduce faecal moisture content to 60%, sheep to 50% and camels to 45% (Macfarlane, 1964). The observation that the faeces of zebu cattle contain less water than do those of European cattle in the same dietary and environmental conditions (Quartermain et al, 1957) may explain in part the lower water requirement of the zebu (Phillips, 1960). Even in donkeys, faecal water content seldom drops below 60%, so that in this species and cattle, one third to one half of the total daily water loss is in the faeces (Schmidt-Nielsen, 1965).
Table 9. Average composition of milk from indigenous arid-adapted Ethiopian livestock and temperate-type cattle.
|
Constituent |
Milk composition (%) |
||||
|
Barka cattle |
Adal goats |
Adal sheep |
Adal camels |
Temperate-type cattle |
|
|
Moisture |
86.1 |
88.2 |
86.4 |
85.6 |
87.6 |
|
Ash |
0.6 |
0.6 |
0.6 |
0.9 |
0.7 |
|
Protein |
3.8 |
3.3 |
4.4 |
4.5 |
3.2 |
|
Ether extract |
5.0 |
2.9 |
4.1 |
5.5 |
5.4 |
|
Carbohydrate |
4.5 |
2.8 |
3.7 |
3.4 |
4.8 |
Source: Knoess (1977); Williamson and Payne (1978).
Milk production represents a severe drain on the water resources of an animal. The water turnover of lactating camels and sheep in a hot, Australian environment has been measured at 44% above that of non-lactating animals (Macfarlane and Howard, 1972), while dairy cattle in the tropics require an extra 3 l of drinking water for every litre of milk produced (Barrel and Larkin, 1974). However, the moisture content of the milk of arid-adapted ruminants is not very different from that of other livestock (Table 9), probably because the young suckling animal needs water as much as nourishment from milk.
3.3.1 Drink
3.3.2 Water in food
3.3.3 Guttation, dew and hygroscopic plants
3.3.4 Respiratory and cutaneous water intake
3.3.5 Metabolic water
3.3.6 Milk
3.3.7 Faeces and urine
The provision of drinking water for livestock is the main concern of this series of research reports on water and livestock. Accordingly, mean daily water requirements are given in Table 10. The problem is that drinking is only one of a number of avenues of water intake, and it may not even be the largest one. The relative importance of the different forms of water intake will vary with weather, diet and management, within a species or breed as well as between them.
Mean values for drinking water intake by African ruminant livestock are given in Table 10. However, when planning a water supply, the capacity is usually designed to meet the maximum requirements of the animal, both in terms of its daily requirements and the amount it can drink at one visit. The requirements in practice (Classen, 1977), have been found to be well below the theoretical maximum values obtained by deducting the contribution of respiratory and metabolic water inputs from the maximum body water turnover rates obtained in the field (King, 1979). The explanation is probably that maximum values are associated with the ingestion of a good quality forage with a high rather than low moisture content.
Table 10. Estimated daily drinking water requirements for non-lactating livestock under African ranching conditions.
|
|
Daily drinking water requirement (1) |
|||
|
Species |
Weight (kg) |
Mean |
Theoretical maximum |
Practical guideline for development |
|
Goat |
30 |
2.0 |
5.4 |
5.0 |
|
Sheep |
35 |
1.9 |
5.2 |
5.0 |
|
Zebu bovine |
350 |
16.4 |
56.1 |
25.0 |
|
Camel |
500 |
18.4 |
34.0 |
30.0 (est.) |
Source: Barrett and Larkin (1974); Classen (1977); King (1979).
The maximum amount an animal can drink at one visit to a watering point varies with its degree of dehydration and the time it is allowed to spend near the water. In many parts of East Africa, Classen (1977) found that indigenous zebu cattle drank about 23 l when watered daily, up to 35 l after a very long walk on 2-day watering regime, and a maximum of 45 l on a 3-day regime, but at the risk of water intoxication and death. French (1956b) found that mature zebu oxen on a 2-day watering regime could drink 70 l in 1 h. Following severe water deprivation, cattle, sheep, camels and donkeys can all drink a large amount rapidly (Schmidt-Nielsen, 1965). Macfarlane and Howard (1972) found that camels which were dehydrated by 20 to 25% replaced 60% of the weight lost as water (80 - 1001) in the first drink, while sheep and cattle replaced 75%. The animals replaced all the weight lost from dehydration in 1 or 2 days. Field (1977) observed that camels belonging to pastoralists at North Horr, Kenya drank three times over a period of 2.3 h and assumed that this behaviour was necessary for complete rehydration. However, where there is considerable pressure of stock on a watering point, animals may not be given a chance for a second drink. In such a situation, it is not clear whether the animal learns to drink a very large amount in a short time, or whether body water use is cut back to the smaller amount that can be replaced in one drink. Otherwise, many livestock would become more and more dehydrated as the dry season progressed. After a large drink, livestock often stagger about, then lie down and are left undisturbed for an hour or two before being moved away (R. Sandford, unpublished). Their behaviour may relate to water intoxication, or to physical discomfort resulting from mechanical interference with normal bodily functions caused by a distended rumen.
Water intoxication occurs following ingestion of excessive quantities of water, especially if a great deal of salt has been lost due to severe exercise or high environmental temperatures. The water is absorbed into the bloodstream, reducing the plasma osmotic pressure. This reduction may be sufficient to cause the erythrocytes to swell and burst, resulting in a severe haemolytic anaemia. Cellular hydration occurs, particularly in the brain, causing a condition analogous to cerebral oedema. This results in nervous signs, including muscle weakness, tremor, restlessness, ataxia, convulsions and terminal coma. It may be avoided in a susceptible animal by giving limited access to water at the first drink (Blood and Henderson, 1960).
Severe water intoxication is uncommon in ruminant livestock indigenous to the dry regions of Africa. They have developed mechanisms to cope with it, similar to those described for the camel and the Bedouin goat. In these last two species, large volumes of drinking water are retained in the rumen until the osmolality has been raised with urea and electrolytes from the extracellular fluid, especially via the saliva, and also from desert plants with a high salt content. Absorption of water is slower from the rumen than it is further down the alimentary tract. Thus the osmotic stability of red blood cells in camels, zebu cattle, and certain haired smallstock is probably never challenged (Choshniak and Shkolnik, 1978). The same appears to be true of donkeys, even though their entire alimentary tract may be flooded when they drink (Maloiy and Boarer, 1971).
McDowell (NRC, 1980) describes another form of water intoxication in studies of sheep in a hot room. Under these conditions, one in five sheep started to consume very large volumes of water with a corresponding reduction in feed intake. These sheep died as a result of starvation, due to the substitution of water for gut fill. A similar proportion of range sheep brought into feed-lots in Iran died for the same reason.
Animals that are too weak to drink may be rehydrated with isotonic fluids containing appropriate electrolytes (0.60% sodium chloride, 0.27% lactate, 0.04% potassium chloride and 0.02% calcium chloride). Oral administration is satisfactory provided gut absorption is normal. Otherwise the intraperitoneal route is preferred because a large intravenous injection may cause cardiac embarrassment. An adult bovine may be given 4 l of isotonic solution intravenously in 30 minutes without untoward effect (Blood and Henderson, 1960). Although this amount may represent nearly 20% of blood volume, it makes less than a 2% contribution to the total body water pool.
The quality of drinking water is often as important as the quantity. Water quality is affected by total soluble salt concentration, the presence of some salts specifically toxic to animals even in low concentrations, and possible contamination with disease-producing micro-organisms or their spores.
High evaporation rates from lakes and dams in Africa can raise the mineral content of drinking water; borehole water is also frequently brackish (saline). The response of livestock to highly saline drinking water is to increase their water intake, but at a certain concentration the appetite becomes depressed and fluid intake is reduced. The reason is that the higher concentration of salt requires a greater proportion of the water ingested to be used for salt excretion, until not enough water is left for other functions. The salt concentration at which this depression occurs is a measure of arid adaptation in a species, as shown in Table 11. In practice, if animals become accustomed to a salty water supply they can tolerate much higher salt concentrations than if forced to drink the more concentrated solutions without a preliminary conditioning period (French, 1956a).
Table 11. Tolerance of salty drinking water by different livestock species.
|
Species |
% total salts in drinking water |
|
Camel |
5.5 |
|
Goat |
1.5 |
|
Sheep |
1.3-2.0 |
|
Cow |
1.0-1.5 |
|
Donkey |
1.0 |
|
Horse |
0.9 |
|
Pig |
0.9 |
Source: French (1956a), Wilson (1967, Macfarlane (1971); Maloiy (1972).
Table 12. Safe levels of toxic elements and ions in livestock drinking water.
|
Element |
Level (mg. l-1) |
Remarks |
|
Arsenic (as As) |
1.0 |
Inorganic oxide, especially from dips |
|
Boron (as B) |
|
Present at < 4 mg.l-1, whereas 450 mg.l-1 inhibits growth |
|
Cadmium (as Cd) |
0.01 |
Accumulates in liver and kidneys |
|
Calcium (as Ca) |
1000 |
<700 mg.l-1 desirable for beef, esp. if Mg present |
|
Chromium (as Cr) |
1-5.0 |
Industrial effluent, but not readily absorbed |
|
Copper (as Cu) |
0.5-2.0 |
Essential trace element, but could reach toxic level from wide agricultural use |
|
Fluoride (as F) |
2.0 |
See text |
|
Iron (as Fe) |
10.0 |
Scouring caused by grazing pasture irrigated with high-Fe water |
|
Lead (as Pb) |
0.5 |
Cumulative poison |
|
Magnesium (as Mg) |
250-500 |
Predisposes to rickets if Ca content low, sulphate causes scouring |
|
Mercury (as Hg) |
0.002 |
Health hazard to human beings consuming meat |
|
Molybdenum (as Mo) |
0.01 |
Only dangerous if accumulated in (irrigated) pasture |
|
Nitrate (as NO3) |
90-200 |
Sources are deep wells filled by seepage from highly fertile soil, or dams containing much decaying organic matter, e.g. manure |
|
Selenium (as Se) |
0.02 |
To compensate for plant ability to concentrate Se |
|
Sulphate (as SO42-) |
1000 |
High magnesium sulphate causes severe problems |
|
Zinc (as Zn) |
20 |
Natural and industrial contamination, but relatively non-toxic |
Source: Hart (1974).
Salts which cause specific toxic effects are listed in Table 12, together with recommended working levels in drinking water (derived in Australia) which should provide an adequate margin of safety. The amount of information on water analysis is very variable across Africa, but there are some documented cases of toxic ion effects. For example, fluorosis in man and livestock is a problem in volcanic areas and where drinking water is obtained from deep boreholes (Williamson, 1953; Walker and Milne, 1955; Murray, 1967; Said, 1981).
Contamination of stock drinking water with urine, faeces, other animal discharges, or even animal carcasses sometimes occurs in pastoral areas. Examples of bacterial diseases spread by each of these contaminants are leptospirosis, salmonellosis, brucellosis and anthrax respectively. Drinking water is less likely to spread viral diseases, but may be implicated in the spread of foot-and-mouth and rinderpest, among others. A number of stock parasites may spend part of their life cycle in or near water, such as protozoa, flukes, flat-worms and round-worms (BVA, 1976). Because they are frequently introduced via faecal contamination, the faecal coliform level may be used as an indicator of the presence of pathogens. The maximum monthly mean should be less than 1000 organisms per 100 ml or five times that in any one sample (Hart, 1974).
Not much information is available on other contaminants, but these could nevertheless be borne in mind. Blue-green algae toxins have commonly killed livestock in Australia (Hart, 1974), and pesticides such as DDT, which is still used in Africa, could build up in the water of dams or lakes draining agricultural areas, particularly if they do not have large outlets and if they lose much of their water through evaporation.
After deduction of water drunk from total body water turnover, the balance of water intake is made up of water in and on food, inspired and absorbed through the skin, as well as from the oxidation of organic compounds during metabolism. The most important of these non-drinking water sources is water in the vegetation.
During and immediately after the rains the moisture content of grass may be more than 80% and of browse more than 70%. At such high moisture contents many herbivores can go for days without drinking. However, during the rainy season ephemeral water ponds are widespread and so the ability to do without drinking water is not of value. An exception is the grazing of porous, volcanic hills, which hold no standing water and can therefore be exploited by livestock only during the rains, whereas hills of other soil types are usually held in reserve for dry-season grazing. The ability to go without drinking water for days or even weeks, once the ephemeral water has disappeared and the vegetation is starting to dry out, is a priceless asset of some game animals, camels and to a lesser extent desert goats and sheep. The forage moisture threshold at which an animal does not need to drink is illustrated from a study of domestic oryx under African ranching conditions (Figure 4). These conditions are defined as: day-grazing on natural pasture where forage quantity is not limited, drinking water available daily, and penning animals at night. In Figure 4, the theoretical curve of water intake from forage has been constructed, starting with a low DMI (2% bodyweight) at low forage moisture content, and ending with a high DMI (3.5%) for very green grass. This curve has been superimposed on the actual maximum and minimum values obtained for total body water turnover, adjusted by subtraction of estimates of respiratory and metabolic water inputs. High levels of water intake from forage are usually associated with high levels of body water turnover. Nevertheless there are times when, and ways in which, the body water turnover can be kept below the water intake from forage, so that the oryx does not need to drink (chapter 4).
The curve of water intake from forage is similar for indigenous livestock, but their body water turnover rates are higher (Table 13). They will therefore be more dependent on drinking water than the oryx, unless they consistently eat forage with a higher water content. A rough estimate of the moisture content of the diet of each species was obtained by hand grab sampling the plant parts the animals were seen to be eating during each water turnover study (Table 14). The per cent moisture content of the diet of mixed feeders such as smallstock, and particularly dry-season browsers such as eland and camel, is higher than that of grazers such as zebu cattle and oryx. This finding is obvious from measurements, during the dry season, of the water content of herbs and browse which is usually >30%, whereas grass may be <10%. Nevertheless, the contribution of water in browse to body water turnover over the year does not materially alter the ranking of different species' dependence on drinking water, which is similar to their ranking for water turnover (Table 15). The main reason is that the amount of water from forage obtained at dietary moistures <40% is relatively small (Figure 4).
Table 13. Values for adjusteda body water turnover in African game and livestock on a day-grazing regime.
|
Species |
Adjusted water turnover (ml.kg-1.d-1) |
||
|
Minimum |
Maximum |
No. of trials |
|
|
Small East African goat |
62 |
166 |
12 |
|
Eland |
53 |
149 |
15 |
|
Boran zebu |
51 |
150 |
10 |
|
Dorper-type sheep |
50 |
140 |
12 |
|
Camel |
38 |
76 |
5 |
|
Oryx |
21 |
102 |
15 |
a Adjusted by subtraction of metabolic and respiratory water input. Source: Adapted from King (1979).
Table 14. Estimated water content (%) of diet of different species under African ranching conditions.
|
|
Water content (%) | |
|
Species |
Mean |
S.E. |
|
Oryx |
13 |
3.8 |
|
Zebu |
15 |
5.4 |
|
Sheep |
26 |
5.5 |
|
Goat |
29 |
3.8 |
|
Camel |
34 |
1.7 |
|
Eland |
36 |
2.6 |
Source: King (1979).
In arid areas there is usually a variety of juicy plants which would appear to offer livestock an alternative source of water as well as food. Some of these plants are sufficiently palatable to be selected even when there is drinking water available, for instance the juicy herb Commelina and the swollen stem of Pyrenacantha malvifolia (Field, 1975). Other plants such as aloes are only taken towards the end of the dry season. Some bushes with juicy leaves also have a high salt content, which may increase rather than decrease the herbivore's water requirements. For example, it has been found in Australia and the Middle East that sheep more than doubled their water turnover rates when moved from a natural pasture to one high in salt bush (Atriplex spp.) (Wilson, 1966; Degen, 1977a; 1977b). In Africa, game and livestock browse salt bush (Suadea monoica) during the dry season. Whether or not this diet increases their water turnover presumably depends on whether they sweat or pant, and, if they sweat, how much salt is secreted by the sweat glands.
Table 15. Comparison of water turnover and water drunk (ml. kg.-1.d-1) by browsing and grazing livestock
|
Species |
Adjusted turnovera |
Water drunk |
Feeding habit |
||
|
Mean |
S.E. |
Mean |
S.E. |
|
|
|
Goat |
94 |
4.3 |
65 |
4.2 |
Mixed feeder |
|
Eland |
83 |
4.2 |
68 |
3.6 |
Browser |
|
Sheep |
78 |
4.8 |
54 |
4.7 |
Mixed feeder |
|
Zebu |
74 |
5.2 |
47 |
4.5 |
Grazer |
|
Camel |
41 |
2.4 |
37 |
2.6 |
Browser |
|
Oryx |
35 |
3.5 |
29 |
4.7 |
Grazer |
a Adjusted by subtraction of metabolic and respiratory water input.
Source: King (1979).
The contribution of dew and guttation to water intake has been measured on improved pasture in Australia, where sheep obtained between 30 and 130 ml. kg-1.d-1 from these sources (Brown and Lynch, 1972). It would be extremely difficult to measure water intake from dew and guttation under semi-arid conditions on natural pastures. Dew forms nearly every night in some of the semiarid regions of Kenya, but only during the cooler months in real deserts. Wild impala have been observed licking water from short vegetation in the early morning (Lamprey, 1963), and in much the same way sheep deprived of water were observed licking dew off pasture and fences (Brown and Lynch, 1972).
Some plants become hygroscopic when dessicated during the dry season, and may acquire a 20 to 40% moisture content during the night, even without visible dew. Such plants can provide an important source of water for nocturnal grazing animals (Buxton, 1923; Schmidt-Nielsen, 1965). For example, Taylor (1968) estimated that oryx and Grant's gazelle could obtain their water requirements from the desert herb Disperma if they fed in the evening, night and early morning. If the wild oryx does not need to drink when the forage moisture is <40%, then it has either increased its DMI to 3.5% of bodyweight at 40% forage moisture or lowered its minimum body water turnover rate, which is more likely. Figure 4 relates to the domestic oryx on a day-grazing regime. Unfortunately, most domestic animals in Africa have to be penned at night to prevent human and animal predation.
Although there is a net water loss from the respiratory system and through the skin, these two components together make a significant contribution to the input side of the water balance equation. The amount of water (kg) in the inspired air which is exchanged with the water pool accounts for about 10% of total water intake, and may be calculated as follows (Weast et al, 1965):
water in inspired air (kg) =
r.h. × sat. water yap. × resp. min. vol.
where r.h. is relative humidity (%), sat. water yap. is weight of 1 m3 of saturated water vapour (kg) and resp. min. vol. is respiratory minute volume (m3).
The amount of air inspired (m3) was calculated from data on zebu cattle weighing about 220 kg (Finch, 1973) as:
6.64+3,00 f
where f is the breathing rate per minute. Normal values over 24 h for zebu cattle on a maintenance diet are in the region of 110 m3 of inspired air containing 1.4 kg water (King and Finch, 1982).
Cutaneous water exhange was not measured, but it is assumed to be about 10% of the water exchange via the respiratory system (D. Robert-shaw, unpublished). More water is absorbed through the skin if the animal is actually rained on, but cutaneous water intake probably remains insignificant compared with other forms of intake.
The effect of these two components was observed during feeding trials in covered pens on Galana ranch, Kenya. It was noted that livestock reduced their water intake on rainy days, which was explained by their lower requirement for evaporative cooling due to the drop in ambient temperature when it rained (Stanley-Price, in press). However in such a situation there is also a marked increase in the relative humidity and hence the water content of the inspired air.
The oxidation of organic compounds during metabolism leads to the formation of water from the hydrogen present. Attempts to measure this process in fasting cattle based on the dilution of tritiated water were unsatisfactory (J.E. Vercoe, unpublished). Therefore it is still calculated indirectly. An example of the calculation of metabolic water produced from nutrient intake is given in Table 16. Alternatively, the amount of water (g) has been estimated at 0.0294 × total heat production (kJ) (Morrison, 1953).
Besides being an avenue of water gain, there is a generally held belief that the oxidation of fat deposits makes a net contribution to the total body water pool, not just from the hump of the camel and the fat-tail of the sheep, but from deposits in any animal, for example the pig (Skipitaris, 1981). However this assumption has to be qualified by the circumstances (Schmidt-Nielsen, 1965). For instance, metabolic water is a product of oxidation but, in the process of inspiring the oxygen, water is expired. It has been calculated that in a hot, dry environment (ambient temperature 36°C and r.h. 10%) an animal loses 23.5 g of respiratory water in the process of producing 12.3 g of metabolic water (D. Swift, unpublished). As well as water, metabolic heat is generated (418 kJ).
Part of this heat (13.6%) is offset by the heat of vapourisation of the expired water. If the remainder (361 kJ) had to be dissipated by sweating, it would cost 149 ml of water (section 3.2.1). The relationship between metabolic water yield and the water that could be required to dissipate the heat of combustion varies with the organic matter being oxidised. Thus 1 g of fat yields 1 ml of water, but could require 14 ml for vapourisation; 1 g of protein or carbohydrate yields about 0.5 ml of water and could require 6.5 ml of sweat.
Schmidt-Nielsen (1965) has argued that the water of oxidation is only of value where the vapour pressure gradient between the expired air and the environment is shallow, for example in a humid burrow, and when the endogenous heat production is low enough to allow heat storage or dissipation by non-evaporative means? for instance as metabolic rate decreases. Low metabolic rates may be expected to be associated with low water turnovers. Thus the relative contribution of metabolic water to total input is much higher (15 35%) when the rate of turnover (k) of the body water pool is low (k <0.05) than when it is high (k>0.15, metabolic water 5%). For example, penned zebu, eland, haired sheep and goats had a mean rate constant of 0.10 and metabolic water was calculated as 8% of total input, whereas oryx had a rate constant of 0.05 and their metabolic water production contributed 16% (King et al, 1978).
Table 16. Indirect calculation of metabolic water production in ruminants eating a hay/lucerne mixture (g H2O.g-1 DMI)
|
Data required |
Crude protein |
Crude fibre |
Ether extract |
Nitrogen free extract |
Ash |
Total |
|
Amount per g DMI (g) |
0.125 |
0.363 |
0.022 |
0.395 |
0.100 |
1.000 |
|
Digestibility |
0.637 |
0.592 |
0.654 |
0.551 |
- |
- |
|
Amount digested (g) |
0.080 |
0.215 |
0.014 |
0.218 |
- |
- |
|
Water per g oxidised (g) |
0.420 |
0.560 |
1.070 |
0.560 |
- |
- |
|
Metabolic water (g) |
0.034 |
0.120 |
0.015 |
0.122 |
- |
0.291 |
Source: King et al, (1978), based on van Es (1967).
It is recognized in pastoral production systems that the amount of milk left for the calf may not be enough to achieve maximum growth, unless the peak demands of man and the calf happen not to coincide. For example, if the average daily milk yield of a Boran cow is 3.7 kg and only half is available for the calf, then there will be a shortfall of about 1 kg below the figure of 2.9 kg required for rapid growth (Dahl and Hjort, 1976). Thus malnutrition from milk deprivation has come to be accepted as one of the main causes of slow growth and high-mortality of calves in pastoral areas.
However, the water content of milk may be as important to the unweaned animal as its nutritive value. For example, Stephenson et al (1981) measured the daily fluid intake from trough water and milk by free-ranging Merino lambs as 155 ml. kg-1 during the Australian summer. They concluded that milk intake was inadequate to meet the lambs' fluid demands and that insufficient watering points could be a significant factor affecting lamb survival. The daily fluid requirement of the Maasai lamb or Small East African kid, penned in the shade near the boma, or fortified night enclosure, would be lower than that of the free-ranging Merino. However, it is likely to be higher than the mean value for adjusted body water turnover of the adult sheep and goat given in Table 15. Based on data of milk yields in the Small East African Goat (C.P. Peacock, unpublished) and Small East African Zebu (P. Semenye, 1982), values for body water turnover must be: <110 ml. kg-1.d-1 in kids weighing 7 kg at 2 months, and <74 ml. kg-1.d-1 in calves weighing 30 kg at 2 months. The conclusion is that the water requirement of the calf may not be met because its turnover rate associated with optimum growth would be higher than the rate given, which nevertheless demands the peak milk intake of 2.5 kg. d-1.
In Maasailand, suckling livestock are unlikely to be taken to water before they are 1 to 2 months old, and it is not clear whether signs of dehydration are recognized. Where the climate is hotter and drier, as in the extreme north of the Sahel in Mali, unweaned stock, which are kept in camp for 4 to 6 months depending on the species, are given water from a water skin. The amount varies from 1 to 21.d-1 in kids to 5 to 101 every fourth day in camel calves (Swift, 1979).
When milk is the only source of water input (apart from respiratory and metabolic water), then milk intake can be estimated from the turnover of tritiated water in the body of the young ruminant (Macfarlane et al, 1960). However, when this technique of tritiated water dilution was applied to the milk intake of suckling rodents and dogs, the results made little sense until it was found that 50 to 80% of the water lost by the young was recycled by the dam by licking up the urine and faeces. Thus more than 30% of the water secreted in the milk was recycled in the dam (Baverstock and Green, 1975). It is not known if this form of water recycling is important in any large African ungulates.