4.1 Energy production
4.2 Thermoregulation
4.3 Water availability
4.4 Integration of energy metabolism, thermoregulation and water
Different species of livestock have different rates of water turnover, and in general animals adapted to dry environments have lower rates of turnover than those in more temperate zones. Body water turnover is also determined by the size of the animal. The role of water in energy production, thermoregulation and other processes means that there are a number of other factors which affect the turnover rate of body water. These are discussed in detail in this chapter.
One of the main functions of water in the body is as a vehicle for intermediary metabolism. There is a relationship between energy metabolism and body size, derived by Brody (1945) and Kleiber (1947), which is:
F = 0.293 w0.75 (4.01)
where F is fasting metabolism (MJ NE. d-1) and W is bodyweight (kg).
Therefore one might expect a relationship between body water turnover (y) and body pool size (x). This was demonstrated in six species of ruminant in Kenya by the following regression
(Figure 5):
log y = 0.836 log x - 0.619 (r2=0.82) (4.02)
The exponent of x is very similar to that of 0.82 obtained by Macfarlane and Howard (1972) from a wider variety of desert animals.
The reason for the increase in the value of the exponent of body size in equation (4.02) versus equation (4.01) is that water serves another function besides intermediary metabolism, namely evaporative cooling. The effect of having two different power functions is to increase the water to energy turnover ratio with increasing size (Table 17). However, larger animals may exploit water conservation mechanisms related to their bulk, and thus be an exception to the rule (sections 4.2.4 and 4.2.5).
The interrelation between water required for metabolism, evaporative cooling, and total water loss may be expressed in a simple diagram (Figure 6) which links more complex diagrams (Figures 7 and 8). There is a wide range of values for total water loss (Table 18). Moreover, the rate at which water is used for metabolism or cooling is highly variable. The relative importance of the different avenues of water loss has already been discussed (section 3.2). The next step is to try and quantify the effect of the different factors on rates of passage. A model of the heat exchange of the animal with its environment, which calculates evaporative water loss, is in preparation (D.M. Swift, unpublished). When it has been published it will provide a better structure for the contents of section 4.2 on thermoregulation. The next step would be to add energy production (section 4.1) to the model. Neither topic has been addressed sufficiently rigorously in this report to allow modelling except at a very crude level. Nevertheless it does lay the foundation for future work.
Table 17. Daily water turnover versus fasting energy expenditure according to size.
|
Body weight (kg) |
Water turnover (1) |
Energy metabolism (MJ) |
Water to energy ratio |
|
40 |
3.90 |
4.66 |
0.84 |
|
300 |
21.00 |
21.12 |
0.99 |
|
500 |
32.20 |
30.98 |
1.04 |
|
1000 |
77.45 |
52.10 |
1.49 |
Source: Brody (1945); Figure 5.
Table 18. Values for body water turnover in game and livestock on a day-grazing regime.
|
|
Water turnover (ml.l-1.d-1) |
| |
|
Species |
Minimum |
Maximum |
Ratio (max: min.) |
|
Small East African goat |
117 |
302 |
2.6 |
|
Dorper-type sheep |
103 |
278 |
2.7 |
|
Zebu cattle |
97 |
274 |
2.8 |
|
Eland |
90 |
242 |
2.7 |
|
Camel |
53 |
106 |
2.0a |
|
Oryx |
41 |
170 |
4.1 |
a Probably an underestimate due to the small number of trials.
Source: Adapted from King (1979).
Figure 6. Interrelation between energy metabolism. body temperature and total water loss.
4.1.1 Forage intake and metabolism
4.1.2 Starvation
4.1.3 Endogenous heat production
Nutrition exerts a profound effect on body water turnover. There is a significant, positive, linear relationship between faecal output and water turnover in cattle and sheep (Siebert, 1971; Macfarlane et al, 1974). However, the nutritional effects are not constant but depend on the form in which food is acquired, the way in which it is used, and the heat increment of feeding and energy utilisation. The interactions of those effects are shown in Figure 7. Note that the diagram has two components in common with Figure 6, namely body temperature and water required for metabolism, which are the main avenues by which nutrition influences total body water use and loss. The effect of body temperature on metabolic rate can be either positive or negative as illustrated graphically. It is discussed in more detail in section 4.1.3.
The effects of forage intake on body water turnover depend on four major characteristics of the food eaten. The first is the water content of the forage which, in certain situations, has a significant positive correlation with water turnover (King et al, 1975). Very green vegetation can supply all the water needs of the animal and more. Theoretically, plant moisture content may even restrict appetite when it is retained in ingested forage by the sponge effect of coarse structural components (Van Soest, 1982). However, the water is usually released from the forage and is rapidly absorbed from the rumen or passed out in moist faeces. Absorbed water can be excreted from the kidneys of sheep at a rate of 30 l.d-1 so that the DMI of sheep grazing green grass was not limited by its moisture content of 83% (Macfarlane et al, 1966b). Similarly it has been shown that cattle can use grazing with a moisture content of 75 83% very efficiently (R.E. McDowell, unpublished), implying a high body water turnover rate.
Figure 7. Flow chart of intermediary metabolism.
There is a second reason for the significant, positive correlation between vegetation moisture and body water turnover. Green forage usually has a high digestibility and nutritional value, thereby allowing increased DMI and a raised metabolism with its concomitant demands for water (Forbes, 1968; Springell, 1968).
At the other end of the scale, very dry forage will produce a low demand for water for intermediary metabolism, but paradoxically might increase the water needed to dissipate the extra heat increment of feeding, and to facilitate digestion and excretion. For example, if the DMI of grass (of 10% moisture and 40% apparent digestibility) was 2% of bodyweight, then daily water intake from it would be 3 ml. kg bodyweight -1 and faecal water output 27 ml. kg bodyweight-1.
The salt content of the forage also affects water turnover, as discussed in section 3.3.2.
The term metabolism may be applied to the modification of all categories of food digested. However, the total energy intake of the herbivore is usually the most important factor determining total output of animal products, as long as the relatively small protein, mineral and vitamin requirements are met (Williamson and Payne, 1978). This statement may not be entirely true for high yielding dairy cows (Fuquay, 1981), but such animals are not associated with pastoral production systems. The main source of energy from forage is carbohydrate. The fat content of the diet of herbivores is usually very low (1-4%), except in the newborn suckling animal when it provides 30% of DMI and 50% of caloric intake (Van Soest, 1982). Milk intake has already been discussed in section 3.3.6.
Figure 8. Factors affecting environmental heat load and thermoregulation.
Increases in DMI result in increases in metabolic rate and productivity. The extra water requirements for different forms of production have been estimated for farm animals in the British Isles at the highest temperatures experienced there (Table 19). They are included for reference when water turnover is estimated in the pastoral situation (section 5.1.8) for which no such data exist.
A severe reduction in DMI produces a similar reduction in body water turnover. Nevertheless, metabolic processes must continue if the animal is to survive, and so body tissues are catabolised to make up the shortfall in food intake. This tissue mobilisation produces metabolic water as a byproduct (section 3.3.5) and a continuing use of water for metabolism. However, the need is reduced by 20 to 30% in the zebu because of the depression of metabolic rate in response to a diet of half that of maintenance. The resultant reduction in body water turnover by 30 to 40% is therefore an expression of both a reduced DMI and a lowered resting metabolic rate (Finch and King, 1979; 1982).
Endogenous heat is both a by- and an end-product of metabolism, as shown in Figure 7. Its effect on body water turnover becomes important when, combined with exogenous heat, it exceeds the upper critical heat load on the animal, which then has to resort to evaporative cooling. The significance of the upper critical heat load is that at higher heat loads metabolism actually increases, due to the oxygen cost of evaporative heat loss and the increase in metabolism associated with a rise in body temperature (Robertshaw and Finch, 1976). It is depicted in the small graph relating body temperature (t) to metabolic rate (MR) in Figure 7. It is known as the Q10 effect and can be explained by the fact that a rise in temperature accelerates most chemical reactions (Schmidt-Nielsen, 1975).
Although endogenous heat production may contribute less than one third of the total heat load on the animal (Table 5), it may be the portion which can be most easily reduced by the animal, by decreasing its activity and food intake. Walking and feeding account for 90% of the diurnal activity of livestock under African commercial ranching conditions (Lewis, 1975; 1977; 1978).
Table 19. Water requirementsa of farm animals in the British Isles at the highest temperatures experienced.
|
Animal |
Environmental temperature (°C) |
Water intake (1.kg-1 DM eaten) | |
|
1. Beef cattle |
15-21 |
4.1 | |
|
|
21-27 |
4 7 | |
|
|
>27 |
5.5 | |
|
2. Lactating cows |
|
As for 1. with additional allowance of 0.871 water/kg milk produced | |
|
3. Pregnant cows (last 4 months) |
|
1 values × 1.5 | |
|
4. Sheep |
15-20 |
2.5 | |
|
(growing and fattening) |
>20 |
3.0 | |
|
5. Pregnant ewes: |
|
| |
|
|
3rd month |
|
4. values × 1.5 |
|
|
4th month |
|
4. values × 1.8 |
|
|
5th month |
|
4. values × 2.2 |
|
6. Lactating ewes: |
|
| |
|
|
1st 8 weeks |
|
4. values × 1.5 |
|
|
2nd 8 weeks |
|
4. values × 1.25 |
a Water from food and drink.
Source: McDonald et al (1976).
Pastoral cattle often have to walk long distances between grazing and water. But the extra water cost of walking per se is negligible if the solar heat load is moderate. For example, when the total solar radiation was 2140 ± 138J. cm-2.d-1, the cost of walking 16 km instead of 8 km.d-1 was an extra 4.4 ml.kg-1.d-1 for zebu cattle on a half maintenance ration. When total solar radiation rose to 2385 ± 59 J.cm-2.d-1 the water cost of travelling the extra 8 km was significantly higher (P < 0.005), by an extra 11 ml.kg-1.d-1. The reason was that the walking period of the cattle travelling 16 km.d-1 extended into mid-afternoon, when the environment was at its hottest (Finch and King, 1982). The impact and avoidance of high solar heat loads will be considered in more detail in the next section. The present discussion is concerned with the heat produced from walking, which can be considered from two angles: total energy expenditure and heat production over a given distance is least when the zebu cow is encouraged to walk at about 3 km.h-1; however, the rate of heat production will be reduced as speed declines to 1.5 km.h-1, when the energy cost flattens out at 20% above that of standing still, except in the starving zebu, when it appears to go on declining (section 5.1.3). Such control of endogenous heat production may be abandoned by dehydrated cattle when they approach water. Classen (1977) reported that very thirsty and debilitated animals will run to water and may die from exertion and overdrinking. Perhaps the extra exertion precipitates an explosive rise in body temperature (see section 3.1).
The mechanism responsible for a reduction of food intake in heat-stressed animals is not clear (Van Soest, 1982), but its effects are. The reduction will affect the heat increment of feeding, which is about 40 kJ.MJ-1 metabolisable energy (ME) when eating and ruminating fresh herbage on temperate grasslands (Webster, 1980). The sequel to reduced intake will be a reduction in the heat increments of production and maintenance (Robertshaw and Finch, 1976). This drop in heat production may be partly offset by mobilisation of body tissues (section 4.1.2).
Thus the immediate effect of inactivity and inappetence is to reduce endogenous heat production by about 30% and the total heat load in the free-ranging animal by 10 to 20%. The longer term effect of the reduction in ME due to lack of appetite is a reduction in metabolic rate which is related to or caused by a reduction in endocrine activity. Chronic exposure to heat depresses thyroid activity, as well as plasma cortisol and growth hormone concentrations and turnover rates. All three hormones act in cooperation and are calorigenic (Thompson, 1976; Robertshaw and Finch, 1976). Chronic exposure to heat stress also depresses libido and spermatogenesis, or suppresses oestrus and ovulation, and causes luteolysis and embryonic mortality, particularly in temperate breeds of livestock (McDowell, 1972). All these reductions in metabolic activity, and its byproduct endogenous heat production, will reduce body water turnover.
4.2.1 Environmental heat and humidity
4.2.2 Cold and rain
4.2.3 Behavioural response to heat stress
4.2.4 Coat characteristics
4.2.5 Mass, shape and appendages
4.2.6 Body temperature fluctuation
4.2.7 Counter-current cooling
As already indicated, livestock in sub-Saharan Africa use larger amounts of water for evaporative cooling than for intermediary metabolism, to dissipate the high solar energy load. The way in which the residual water available for sweating and panting (Figure 6) contributes to total body heat loss is shown in Figure 8. This diagram shows evaporative heat loss acting negatively, via total heat loss, on the same vertical column of components of body heat gain through to body temperature as in Figure 7, but with the superimposition of the sun on top. Of course, not all heat loss occurs via evaporative cooling, nor is it always hot in the tropics. The way in which thermoregulation affects body water turnover involves a complex interaction of climatic variables with behavioural responses and anatomical or physiological adaptations of the animal.
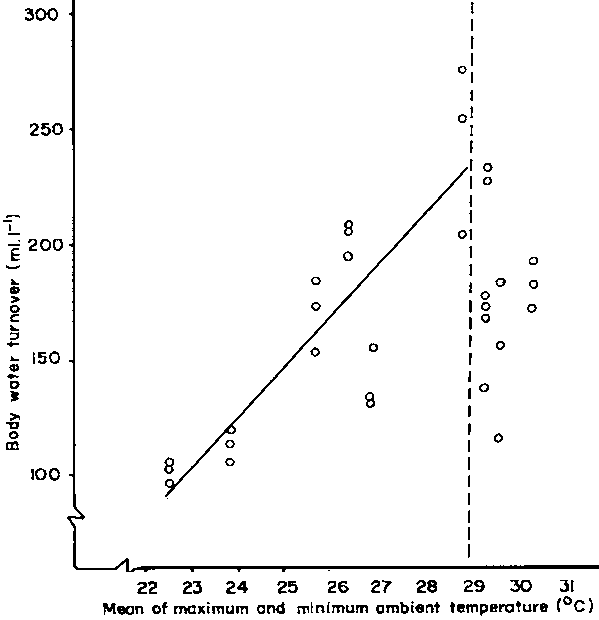
During the daylight hours almost all the heat gained from the environment comes directly or indirectly from solar (shortwave) radiation. Heat is gained from convection, conduction and long-wave radiation only if the temperature of the air and of objects in the habitat is higher than the skin temperature of the animal. The degree to which the habitat heats up varies with its composition. For example, desert sand reflects 30-40% of the incoming radiation, desert shrubs 30-38%, and green grass about 25% (Barry and Chorley, 1971). Although green vegetation reduces the reflectivity (albedo) of the ground, it does not heat up. Instead it acts as a heat sink for longwave radiation, for example from a warm animal, because it is at a lower temperature than its surroundings as a result of transpiration. Thus, even during the day, there is usually a net outflow of longwave radiation from the animal, and this increases at night. In order for this dry heat loss to balance the solar heat gain, the ambient temperature must be low, as it is at high latitudes or high altitudes. There is a 6.5°C drop, or adiabatic lapse rate, for every 1000m increase in altitude. Therefore, if sweating accounts for 21% of the net heat loss of a zebu cow at 1675 m a.s.l. near the equator in Kenya (Table 5), it will account for a much larger proportion at sea level provided the atmosphere remains dry. Because sweating can account for up to 80% of the water used by a ruminant in the tropics, it is not surprising to find correlations between ambient temperature or solar radiation and total body water turnover. For example, on a ranch in Kenya situated 180 m a.s.l. a significant correlation was obtained between daily mean ambient temperature up to 29°C and body water turnover (x) in the zebu (Figure 9), namely:
y = 21.518x - 390 (r2 = 0.71)
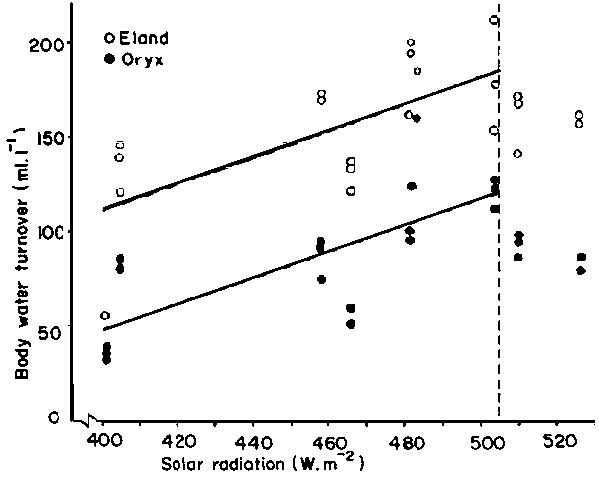
Equally significant relationships (P <0.001) were obtained between direct and diffuse solar radiation and total body water turnover, for example in eland and oryx (Figure 10). It should be noted that the intercept of the regression line in Figure 10 is lower in the oryx than the eland, but that thereafter the response of body water turnover to increasing heat load is similar in both species - a point which will be discussed in section 4.2.3.
Figures 9 and 10 both illustrate that at high heat loads, above 29°C 'mean' ambient temperature for the zebu, or at high levels of solar radiation - 505 W.m-2 for the eland, and 475 W.m-2 for the oryx - the body water turnover rate declines. The regressions have been interrupted at this point to emphasise the phenomenon. Inclusion of plots to the right of the dashed vertical lines or the use of curvilinear regressions does not improve the fit. The explanation for the sudden decline in response would appear to be that the animals dissipate the increasing environmental heat load by increased evaporative cooling only up to a level determined by the body water turnover rate. In eland and cattle this rate approached 18 and 24% respectively, and if exceeded could result in severe dehydration even when drinking daily. The cut-off point of 12% in the oryx is more difficult to explain. It may be associated with less extravagant water cooling mechanisms, and be related to a natural inclination to avoid daily watering even when it is offered. For all species, the reduction in body water turnover rates at higher heat loads than those mentioned above implies that (a) other forms of heat load are being decreased and/or (b) other thermoregulatory mechanisms are being brought into play, or (c) homeothermy is being abandoned (sections 4.2.3, 4.2.6 and 4.2.7).
The value of sweating as a cooling mechanism varies in the different climatic zones of Africa, because the rate at which water evaporates from the body varies inversely with the water vapour pressure in the air. However, at moderate humidities this reduction is much smaller than might be expected. It can be explained by assuming that the rate of sweat secretion is unchanged and an increase in humidity causes a temporary reduction in evaporation and a build-up of moisture on the skin. As a result the vapour pressure at the skin surface increases, automatically re-establishing the vapour pressure gradient between skin surface and air until evaporation is in equilibrium with the rate of sweat secretion. At extremely high levels of humidity there are more marked reductions in evaporation (Thompson, 1976). Such high levels of vapour pressure can occur, both in the humid tropics and in housed livestock, where they may be the main cause of variation in heat stress and body water turnover rather than the relatively small seasonal fluctuations in ambient temperature (Kamal and Seif, 1969; Siebert and Macfarlane, 1969; Kamal and Johnson, 1971).
Figure 9. Relation between daily total body water turnover and mean ambient temperature in zebu cattle under African ranching conditions. Source: J.M. King (unpublished)
The combined effect of temperature and humidity has been developed into an index (THI) by the United States Weather Bureau to describe discomfort in man. The index appears to be applicable to animals (McDowell, 1972):
THI = 0.72 (ºCdb + ºCwb) + 40.6
where
db = dry bulb temperature in °C, and
wb = wet bulb temperature in °C.
When this index was applied to Holstein cattle at the University of Missouri by Johnson et al (1963) it was found that cows appeared comfortable when the index was below 70, but there was reduced milk yield and food intake above 75; all age groups showed measurable degrees of discomfort at an index value of 78 and above. These findings from North America are relevant to a number of dairy projects in the coastal areas of West Africa, where potentially high-yielding Holstein cattle are producing barely enough milk to support their calves, despite expensive housing. The cattle are often thin and their lack of productivity is blamed on such factors as poor nutrition and intermittent water supply. However, when the THI values for adjacent areas, calculated from climatograms published by Pagot (1974), are plotted on a monthly basis and related to the response observed by Johnson et al (1963), it becomes apparent that temperate dairy breeds kept in these areas suffer from heat and humidity stress for much of the year (Figure 11).
Figure 10. The relation between daily body wafer turnover and solar radiation in domestic Bland and oryx under African ranching conditions. Source: King et al (1975).
Although most of the discussion in this report is concerned with heat stress, in the northern and southern African deserts (in winter) and in the highlands (every night) the ambient temperature falls sufficiently far below the animal's critical body temperature to stimulate thermogenic heat production. Rainfall accelerates heat loss from the animal by increasing the conductivity of the coat. The result is an extra demand on the nutritional intake and reserves of the animal, which may be sufficiently important to influence the type of animal selected by the pastoralist as well as its management (sections 4.2.4 and 7.2.1).
Thermogenic heat production causes a slight increase in body water use. However, this is accompanied by a reduction in insensible water loss from the skin as a result of peripheral vasoconstriction, as well as increased condensation of saturated expired air in the respiratory passages which have been cooled by the cold inhaled air (Schmidt-Nielsen, 1975). Thus the net effect of cold ambient temperatures is to reduce water loss.
The activity of herbivores on tropical rangeland is a mixture of vital activities, such as feeding and walking to water, plus 'behavioural amelioration' (Macmillan, 1972) of a frequently hostile environment. The resultant compromise gives the typical activity pattern of herbivores on a day-grazing, night-enclosure regime (Figure 12). Herbivores with access to night grazing may be expected to spend a greater proportion of the day idle or in the shade, whereas those that are confined at night can only avoid high heat loads at the expense of feeding time (Lewis, 1977; 1978).
Shade seeking is one of the more conspicuous forms of behavioural amelioration. As the dry season progresses, wild impala have been observed to move lower down the catena on which they graze, moving to where the vegetation is greener and the shade is deeper (Jarman, 1973). In the absence of shade, sheep will stand with their heads under each other's bellies; this manoeuvre may serve the dual purpose of avoiding overheating the hypothalamus and the attentions of the nostril fly (Oestrus ovis).
Wallowing is also a useful behavioural adaptation, allowing an animal to use evaporative cooling without any loss of body water. A high rate of evaporation can be sustained for more than 2 h if the animal wallows in mud rather than water (Ingram, 1965).
The structure and colour of an animal's coat will affect the flow of energy and water across ¿he skin (Hutchinson and Brown, l 969).
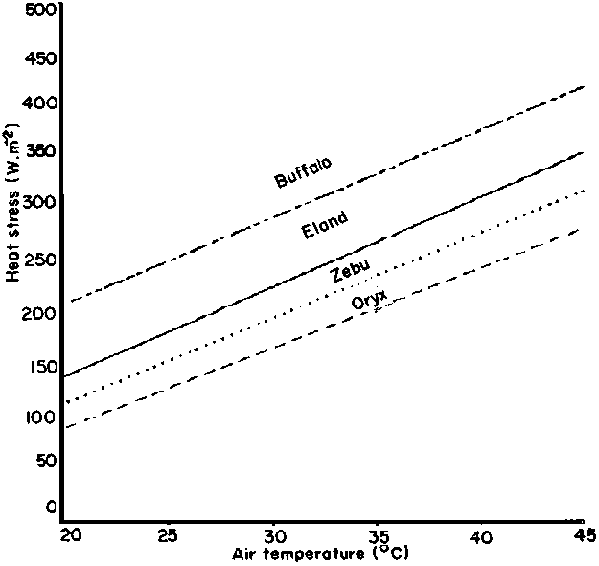
The heat stress on the animal from the radiant environment has been defined as the inward sensible heat flow at the base of the coat (Finch, 1972a). This heat stress on the skin has been calculated by Lewis (1977) in four African ruminants at varying-air temperatures for a constant radiation load (Figure 13). Because the intercepts of the regressions were different, he concluded that the hair barrier to solar heat was different. The greatest heat stress occurred in the African buffalo, with its sparse black coat. Next was the eland, which has a coat colour with a low absorption coefficient (0.75) similar to the brown zebu (0.78) but a coat structure which is sparse and short (hairs 0.22 mm long) compared with the cow (0.30 mm long) (Finch, 1972b; 1973). The least heat stress occurred in the oryx, which has a coat colour with an absorption coefficient of about 0.65 (Stewart, 1953) and a coat structure which is dense and long (hairs 0.4 mm long) (V.A. Finch, unpublished). The fact that the slopes of all four regression lines were parallel implied that convective heat loss was essentially the same in all species and is directly related to air temperature.
The heat arriving at the skin surface makes thermoregulatory demands on an animal, and the physiological response of evaporative cooling by sweating or panting increases the water turnover. For example, it has been found that shearing Merino sheep during the Australian summer doubled their water turnover compared with that of unshorn sheep (Macfarlane et al, 1966b). Similarly, the lower body water turnover of oryx compared with eland at a given solar radiation load (Figure 10) could be ascribed not only to superior water conservation mechanisms but also to the different characteristics of the coat.
Figure 13. Predicted relationship between heat stress on the skin and air temperature in African ruminants with different coat characteristics. Source: Lewis (1977)
Within a species which has a variety of different coat colours, the effect of colour can be considerable. For example, Finch and Western (1977) showed that the inward sensible heat flow at the base of the coat was greater in black cattle than in brown, where in turn it was considerably greater than in white cattle (Table 20). Water drunk, on a daily watering regime, followed a similar pattern to radiation absorbed. When drinking was restricted to every 2.5 days, all colours drank the same amount, which was to the limit of their gut capacity. During the days between drinking the dark cattle became dehydrated more rapidly, their body temperatures rose more, and their appetites were depressed more than in the light coloured cattle.
The same authors point out that dark coat colours become an advantage with increasing altitude and decreasing air temperatures, which may drop to 5°C at night in certain pastoral areas of Kenya. In such situations the local zebu cattle have to expend energy on thermogenesis because they are poorly insulated and not physiologically adapted to a cold climate. When the sun rises, black cattle rapidly absorb solar radiation, whereas white cattle continue to waste chemical energy on thermogenesis to keep warm. At the end of the dry season, if animals are starving, coat colour may make the difference between life and death.
Table 20. Mean values (±SE) of sensible heat flow (W.m-2) towards the animal for l black, 1 white and 2 brown steers for 3 days measured between 09.00 and 15.00h at 1430 m a.s.l. in Kenya.
|
Cattle colour |
Radiation absorbed |
Sensible heat loss |
Inward sensible heat flow |
|
Black |
691 ± 14 |
573 ± 7 |
118 ± 24 |
|
Brown |
630 ± 6 |
531 ± 7 |
99 ± 17 |
|
White |
549 ± 14 |
499 ± 8 |
50 ± 15 |
Source: Finch and Western (1977).
Finch and Western (1977) found a number of field situations where cold and heat appeared to exert a strong selective pressure on cattle coat colour in Kenya, and concluded that the relationship was clearly recognized by the pastoral tribes. For example, in a country-wide survey of cattle colours they found a linear decrease in the proportion of light coloured cattle in the herd with altitude, and an increase with predicted heat stress and potential evaporation, these last two environmental parameters being closely correlated. The relation between per cent light cattle per herd (y) and potential evaporation (x) was:
y = 0.07x-73.9 (r2 = 0.82) (Figure 14).
During the droughts of 1973 and 1975, significantly more white than black cattle died in the Amboseli area of Kenya, where dark cattle predominate and night temperatures fall below 10°C. However at Hola, in the hot dry country of eastern Kenya where white cattle predominate, the Orma herders reported that proportionately more black than white cattle die during droughts.
Coat characteristics which are favourable for evaporative heat loss may be different from those which provide insulation from the environmental heat load. Under dry conditions, sweat evaporates at the skin surface and the hair insulation encourages heat to be drawn from the skin and not the air (Schmidt-Nielsen, 1965; Edney, 1966). Thus evaporation of sweat under a thin layer of insulating hair is probably the best physical compromise in allowing the skin to lose heat while providing protection from high solar radiation (Macfarlane, 1964). In the hot humid tropics, where high ambient temperature and relative humidity may contribute more to heat stress than solar radiation, a thick coat may not be an advantage. The sweat evaporates more slowly than it accumulates and the hair becomes wet. One can speculate that as a result environmental heat is conducted through the wet coat to the skin surface, while evaporative heat loss occurs at the tip of the hairs.
Woolly coats can be an advantage in animals that pant to lose heat. There is no doubt about the advantage of wool as an insulator against the cold, and McDowell (1972) has suggested that wool is an advantageous covering in a hot dry climate, but not in a humid one. The reason given is that wool growth is usually associated with more sebaceous secretion than hair, and the resultant oily coat absorbs less radiation and also tends to retard evaporation from the skin. However, woolly coats are not a feature of smallstock in sub-Saharan Africa, where Wilson's 'rule' tends to apply - namely that coats are hairy rather than woolly in hot regions (Edney, 1966). However, exceptions can be found, such as the woolly Macina sheep indigenous to the hot, humid Niger delta in Mali. Haired sheep and goats sweat considerably as well as pant to lose heat, although they can reduce cutaneous evaporation when dehydrated (Maloiy and Taylor, 1971; McDowell, 1972).
There are a number of other coat characteristics that have not been considered, such as reflectance rather than colour (e.g. black is often shiny), and protection of skin against photosensitisation. However, the subject of coat characteristics has probably been discussed as much as is warranted here because, in some instances, the coat may be little more than a phenotypic expression of other physiological adaptations to a particular environment. For example, the fawn coat colour of African donkeys looks as if it might contribute to heat tolerance, but the coat structure is thin and no marked temperature gradient has been measured between the outer hair surface and the skin (Bullard et al, 1970).
Figure 14. The relation between proportion of light-coloured cattle in herds and potential evaporation in Kenya.
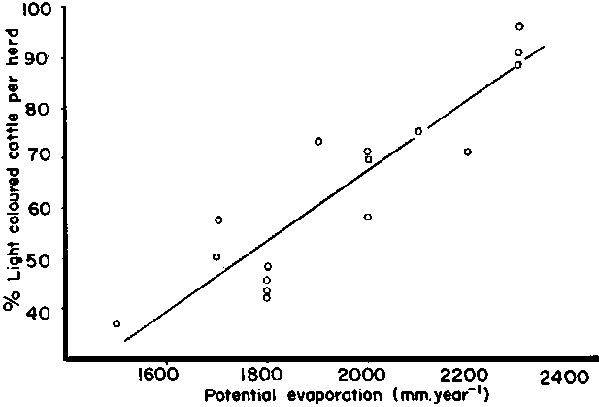
Source: Finch and Western (1977)
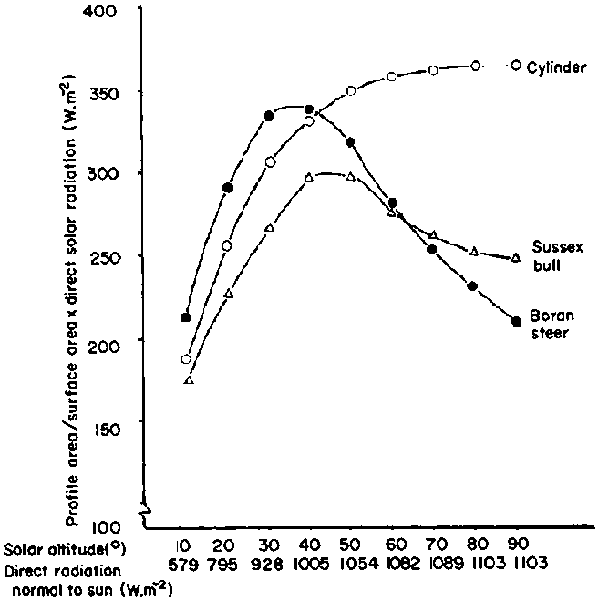
Although the outward appearance of an indigenous animal cannot be used in isolation from its internal physiological adaptations to explain its suitability for particular environment (McDowell, 1972), external body characteristics do play a role in the interaction of the animal with its environment. Their effect on body water turnover is exerted primarily through their influence on the heat exchange of the animal. For example, a large body mass may be an advantage in absorbing residual body heat, but if the sun is the main source of heat load, the less direct sunlight that is absorbed by the body the better. Therefore the profile area at right angles to the solar beam should be as small as possible, particularly at solar altitudes between 40 and 90° when radiant intensity is reaching its peak. At these higher solar altitudes it has been shown that the profile areas of cattle decrease in proportion to their total surface area at a rate greater than radiant intensities are increasing. This results in a decrease in heat absorbed at higher solar altitudes (Finch, 1976). For a cylinder, the ratio of the profile area to total surface area remains constant, and the radiation absorbed continues to increase at higher solar altitudes (Figure 15). Thus at midday the direct solar radiation absorbed by a Boran steer is only 58% of that absorbed by a cylinder. The radiation absorbed by a Sussex bull has been shown to be 17% more than that absorbed by a Boran steer. This difference may be partly explained by the fact that the entire male has a thicker neck and shoulders than the steer, but it may also be due to the Sussex having a broader and longer back than the razor-backed zebu.
A number of hypotheses have been formulated concerning the effects of climate on the mass and shape of animals. Bergson's rule states that similar or related animals are smaller in warm regions than in cold ones, and Allen's rule that the peripheral parts of animals in hot regions are extended. Mount (1979) has shown that pigs reared at 5°C had shorter limbs, smaller ears and were more hairy than their litter mates reared at 35°C. Edney (1966) suggested that the advantage of long legs, neck and ears is to present a greater surface area for convective cooling.
Figure 15. Variation in the amount of direct shortwave radiation absorbed at different solar altitudes by a cylinder. a Sussex hull anti a Boran steer. (Source: Finch (1976), which includes data from Riemerschmid (1943)).
In sub-Saharan Africa, another generalisation seems to apply - namely that wild and domestic ungulates are smaller in the hot, humid regions than their conspecifics in the hot dry savannas (Dorst and Dandelot, 1970; ILCA, 1979a; 1979b). Pagot (1974) noted an association between the volume of a cow's body and the surface and position of the climatogram for the place in West Africa where it had evolved. The salient features of the climatograms have been summarised in Table 21, which shows an inverse relationship between bodyweight or height against atmospheric humidity at similarly high mean ambient temperatures. The places referred to in the table are the same as those for which the THI has been plotted in Figure 11. The conclusion is that, notwithstanding the accepted influences of genetics and disease, the evolution of dwarf breeds may also be related to a chronic hormonal response to an environment in which it is difficult to dissipate heat (see also section 4.1.3).
The indigenous Bos taurus breeds of West Africa have been displaced from all but the tsetse fly belt of Africa by zebu cattle and their established Sanga crossbreeds with the pure Bos taurus. The reasons behind the success of zebu will be examined further in section 7.3.2. The conspicuous features of mass, shape and appendages of the animal have been assumed to have something to do with its more successful adaptation to drier environments. However, the experiments carried out to test this hypothesis have proved difficult to evaluate, particularly when they do not relate to the kind of environment in which the zebu normally thrives. An example of such an environment on Galana ranch in Kenya is given in Table 22, and will be referred to in the ensuing discussion.
Table 21. Height and weight of various races of West African cattle and the climate where they evolved.
|
|
|
Adult cow |
|
|
Mean temp. |
Mean relative humidity | |||
|
Species |
Breed |
Withers height (cm) |
Weight (kg) |
Place |
Altitude (m a.s.l.) |
°C |
SE± |
% |
SE± |
|
Bos indicus |
Zebu |
>125 |
350 |
Tahoua,Niger |
386 |
28 |
1.3 |
38 |
7.4 |
|
Bos taurus |
N'Dama |
110 |
280 |
Labé, Guinea |
1025 |
23 |
0.5 |
66 |
5.2 |
|
Bos taurus |
N'Dama |
105 |
260 |
Odienné, Ivory Coast |
432 |
27 |
0.4 |
70 |
4.4 |
|
Bos taurus |
Savannah WASa |
95 |
200 |
Bouaké, Ivory Coast |
376 |
27 |
0.3 |
75 |
2.3 |
|
Bos taurus |
Dwarf WAS |
85 |
170 |
Cotonou, Dahomey |
167 |
28 |
0.4 |
83 |
0.8 |
a WAS = West African Shorthorn.
Source: Pagot (1974); ILCA (1979a; 1979b).
The larger size of the zebu (especially Boran cows weighing 400 kg) is appropriate to the hot, dry Galana environment. They are reputedly better walkers than Bos taurus breeds, but it is unclear if this attribute has been tested or if zebu have proportionately longer legs. The main heat load is from solar radiation and so the narrow, short profile to the sun is relevant, particularly when examining skin area in relation to size. According to Brody (1945) and Macfarlane (1964), Bos indicus cattle have a greater surface-area to mass ratio (12 - 20%) than Bos taurus. However, Branton et al (1966) found that the differences were more apparent than real in some zebu cross-breds, especially after adjusting for weight at constant age, because what some Bos taurus cattle lack in appendages they make up for in extra body length. The appendages of the zebu, notably the dewlap and navel fold, are poorly vascularised and not adjacent to the main sources of heat production in the body, which reduces their value as radiators and convertors (McDowell, 1972). On the other hand, the location of the skin folds on the dependent parts of the neck and trunk increases surface area without increasing exposure to the sun at midday.
Table 22. Example of an environment in which zebu cattle thrive: Galana ranch, Kenya, 180 m a.s.l.
|
Environmental factor |
Mean |
Maximum |
Minimum |
|
Annual rainfall (mm) |
500 |
1000 |
150 |
|
Annual potential evaporation (mm) |
2200 |
2500 |
2000 |
|
Daily solar radiation (W.m-2) |
500 |
600 |
400 |
|
Ambient temperature (°C): |
|
|
|
|
daily maximum |
33.5 |
37.9 |
28.2 |
|
daily minimum |
21.0 |
23.7 |
14.2 |
|
daily range |
12.5 |
16.6 |
8.8 |
|
Daily wind speed (km.h-1) |
8.7 |
12.8 |
4.7 |
|
Values at 15.00h for: |
|
|
|
|
ambient water vapour pressure |
2.5 |
4.2 |
1.9 |
|
THI |
81.3 |
86.5 |
76.7 |
Source: Woodhead (1968); Bille and Heemstra (1979); J.M. King (unpublished).
The sweat rate on the dewlap was 70% lower than elsewhere (McDowell, 1972). The density of sweat glands was also 36% lower on the dewlap than on the side of Sindhi and Sahiwal cattle, but still 10% more than on the sides of Jersey, Friesian and Red Poll cattle (Nay and Hayman, 1956). Sweating is undoubtedly an efficient way of cooling for the zebu, because the ambient vapour pressure is usually low (Table 22). Yet, in an environment in which water is severely limited for much of the year, it seems unlikely that cattle could maintain high sweating rates for very long without suffering severe dehydration (section 3.2.1). Radiant heat loss is probably the main component of loss because the mean temperature gradient is from the animal to the environment. It is augmented by convection, particularly in the late afternoon when wind speed is at its highest (Tables 22 and 23).
Table 23. Rectal temperatures (°C) of hydrated livestock in Africa.
|
Species |
Rectal temperature (ºC) |
|
Camel |
36.0-39.3 |
|
Horse |
37.5 -38.3 |
|
Cow |
37.6-39.0 |
|
Pig |
38.3-39.3 |
|
Sheep |
38.3-40.5 |
Source: Schmidt-Nielsen (1965), MacKenzie and Simpson (1971); Degen (1977c).
The ears of zebu cattle are larger than those of European breeds but, although they are very vascular, their surface area in relation to total body surface area is small (2%), making them of questionable significance in terms of overall heat loss capability (McDowell, 1972). However, the capacity of the vascular bed and the blood flow can be increased by vasodilation and arterio-venous anastomoses, which have been found in the ear of the calf (Goodall, 1955). Peripheral vasodilation is important in allowing heat flow to the skin surface for evaporative, radiative and convective heat loss (Thompson, 1976). For these last two avenues of heat loss to be effective there must be a marked temperature gradient between the ear and the environment, which is not usually present during the heat of the day. Animals such as the elephant, which use their ears for cooling, do not dilate the blood vessels and increase blood flow in the ear until about 18.00h. Then the temperature in the ear becomes higher than that of the general body surface as well as the environment (Hiley, 1975).
There is one other conspicuous appendage, namely the hump, which increases in size as a zebu improves in condition. It has been postulated that localised storage of fat may reduce the interference of fat layers over the body with the dissipation of heat (Schmidt-Nielsen, 1965). On the other hand insulating layers of fat could work both ways, and need not interfere with the transference of heat from the body core to the surface via the cardiovascular system. Looking at the hump in terms only of heat exchange and energy storage may be wrong, because it contains a considerable amount of lean and fibrous tissue which could relate to the zebu's role as a draught animal in its places of origin in Asia (McDowell, 1972), a role which it still occupies in parts of Africa, particularly Ethiopia.
An attempt was made to assess the collective value of these appendages for heat loss by comparing the response to heat stress of intact zebu (Red Sindhi) bulls with those from which the dewlap, hump and about 10 cm of each ear had been removed (Branton et al, 1966). The authors concluded that there was no justification for the popular association of a superior heat loss capacity with the external characteristics typical of zebu breeds. However it is difficult to relate their experiment to the real world in which the African zebu is to be found. The thermal stress applied in the laboratory was a temperature of 35 - 40°C and a relative humidity of 60% for 6 h. In such conditions the temperature gradient from the animal to the environment could be reversed, and conductance of heat into it would then be reduced by vaso-constriction (Schmidt-Nielsen, 1965; 1972). The appendages would therefore have been a disadvantage unless they were the prime site for sweating, which they were not. The THI in the laboratory was much higher, at 92.8, than that on Galana ranch, which was 81.3. The latter value is high enough to cause discomfort in temperate-type cattle (Figure 11) but the THI is not the most appropriate description of the main heat load, which is from the sun, for which the size, shape and appendages of the zebu may be adapted. One of the two criteria taken to indicate susceptibility to heat stress,- namely raised rectal temperature and respiratory rate, is also suspect because it fails to take into account the value of a labile body temperature (section 4.2.7) in a natural environment with a reasonable diurnal temperature range (Table 22) and a shortage of water.
The zebu cow may not be the best example of adaptation of mass, shape and appendages to the natural thermal environment by African livestock. The preceeding discussion could have focused on other long-legged, razor-backed desert livestock with fat humps, rumps or tails. For example, the large, pendulous ears of the black-haired and black-skinned Nubian goat look particularly functional: the dorsal surface of the ear is covered with short grey hairs, presumably to reduce absorption of solar radiation.
All mammals must maintain a fairly stable deep body, or core, temperature in spite of fluctuations in the environmental temperature and the animal's activity. However, different species and breeds tolerate slightly varying ranges of core temperature, and this characteristic may be related to environmental heat tolerance. Marked departures from the core temperature are associated with extremes of ambient temperature, disease, injury or the administration of drugs (Mount, 1979).
In a moderate African climate, most hydrated ruminants show a diurnal fluctuation in core temperature of 1.5 to 3.9°C (Bligh and Harthoorn, 1965). This diurnal fluctuation is due to some heat storage during the day and heat loss at night. Any further rise in core temperature is accompanied by an increase in evaporative heat loss and some depression of endogenous heat production. A drop in core temperature causes energy expenditure as thermogenesis.
East African sheep and goats are examples of animals with a narrow range in core temperature, i.e. they are obligatory homeotherms. These species appear unable to increase their range of diurnal body temperature by more than 1-2°C in response to heat stress (Maloiy and Taylor, 1971). Instead, they maintain a relatively constant body temperature by panting as soon as they are subject to heat stress. Water intake in African sheep has thus been related directly to respiration rate (Quartermain, 1964), and it has been assumed that the heat tolerance of both species depends on the availability of water to support heat loss by evaporation (Bligh, 1972). Degen (1977c) has pointed out that sheep have relatively high rectal temperatures of 38.7 to 40.5°C, which implies that they absorb less environmental heat and use less evaporative water than might have been expected.
African donkeys also have a narrow range of core temperature, from 37 to 39°C in fully hydrated animals. Under heat stress, they keep their core temperature within this range by evaporative cooling, with a sweat rate 2.5 times that of camels (Schmidt-Nielsen, 1965).
By contrast, the variability in core temperatures is particularly wide among N'Dama cattle. During March in the Gambia, when the days are hot and sunny and the nights are cool and windy, the mean rectal temperature of 10 N'Dama cattle tethered in the open with ad lib food and water was 35.9 ± 0.77°C in the early morning and 39.7 ± 0.84°C in the late afternoon, giving a range of 3.8 ± 0.84°C with or without trypanosome parasitaemia. Extreme values were 34.4 to 41.1°C, with a range of 6.7°C in one animal, apparently related to greater exposure to solar heat load (Greig and McIntyre, 1979). The N'Dama requires far less water than the zebu (Pagot, 1974), probably as a result of this high thermolability.
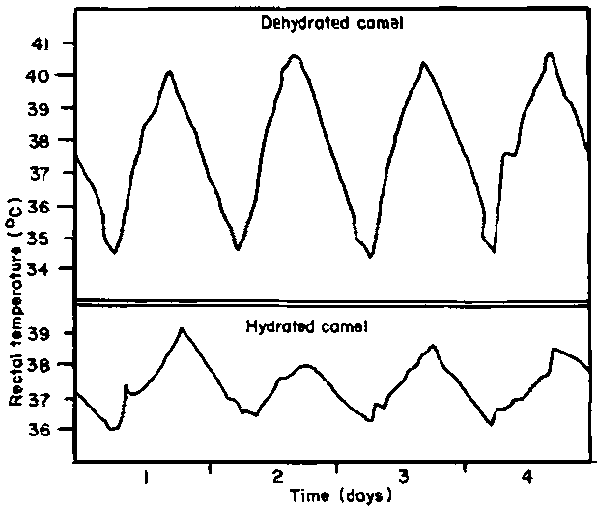
When an animal is dehydrated there is often a further extension of the thermoneutral zone by an upward shift in the threshold temperature for evaporative heat loss and a downward shift in the threshold temperature for heat production (Bligh, 1972). The best known example of this increased diurnal core temperature fluctuation in response to dehydration is in the camel (Figure 16). The animal saved about 1.3% of its body water pool by allowing its body temperature to rise from 34°C to 41°C during the day and disposing of the stored heat at night. An important reduction in heat gain from the environment was also achieved at high body temperatures due to the reduced temperature gradient. The combined effect of these two factors was observed to reduce heat dissipated by a 260 kg camel during the 10 hottest hours of the day in the Sahara to 380 kJ, compared with 1265 kJ in the hydrated state. Water lost by evaporation was reduced from 9.1 to 2.81, that is from 4.7% to 1.4% of the body water pool (Schmidt-Nielsen, 1965).
More recently, loss of homeothermy et night has been demonstrated as a response to starvation. Boran steers on daily watering but half-maintenance rations allowed their body temperatures to drop to 36.8°C compared with 37.8°C in steers on a maintenance ration. The saving in thermogenesis was estimated as 740 kJ, without which the starving cattle would have had to raise their metabolic rate 1.3 times. There was also a lowered sweat rate, so that the body temperature rose rapidly during the morning but was then regulated at the upper range for well fed cattle. The advantage of regulating heat storage in this way lay not in conserving water (although water loss was reduced), but in maintenance of body temperature at an optimum level for metabolic efficiency (Finch and King, 1982).
Figure 16. Daily temperature fluctuation in a hydrated and dehydrated camel. (Source: Schmidt-Nielsen (1965))
Thermolability is exploited in different ways by African game animals, according to their size. The core temperature in an eland can be as low as 32.8°C in the early morning. It takes a lot of solar energy to raise the body temperature to nearly 40°C, when evaporative cooling is initiated. In small animals such as the gazelle, weighing 10 to 60 kg, the body heats up too quickly in the sun for the low early morning temperature to conserve much water. Instead, the upper critical temperature is allowed to rise quickly from 40°C to about 42°C without initiating panting or sweating, thereby reducing the temperature gradient to the environment. The oryx, a desert dwelling ruminant of intermediate size weighing about 150 kg, employs both mechanisms (Taylor, 1970a; 1970b).
The term counter-current cooling refers to the flow of blood in opposite directions in contiguous blood vessels. The heat in the arterial blood, which is coming from the body, is transferred to venous blood from the surface, so that the arterial blood is cooled before it reaches the brain.
The lethal limit to the rise in core temperature is about 6°C above the normal maximum, and depression of central nervous activity, particularly in the respiratory centre, occurs before that (Schmidt-Nielsen, 1975). Yet rectal temperatures as high as 46.5°C have been observed in running antelopes, and may be a normal response. Overheating of the brain in such circumstances is prevented by selective cooling of its blood supply. In ungulates this supply comes primarily from the external carotid artery, which passes through a pool of venous blood at the base of the brain where it divides into a rete (network) of fine vessels. The venous blood comes mainly from the rich submucosal capillary network of the nasal turbinate bones. These capillaries have been cooled by evaporation from the walls of the nasal passages as the animal breathes (Figure 17). It follows that selective cooling of the brain is probably better developed in ungulates that pant rather than sweat to lose heat. Nevertheless, the carotid rete has been demonstrated in domestic bovids and the oryx, and may be called into play when the animal is dehydrated (Daniel et al, 1953; Taylor, 1969). Apart from saving water by limiting evaporative cooling to the brain and not the whole body, the amount of water vapour lost may be reduced by recondensation in a well developed nose. Hoppe (1977) observed that droplets of water appear at the nostrils of the dik-dik, and that these are immediately licked off and swallowed. He also remarked that the trunk-like nose is longest in Gunther's dik-dik, which lives in the hottest areas inhabited by these species.
aCounter-current cooling of arterial blood on its way from the heart to the brain occurs in the cavernous sinus, where the carotid artery ramifies into hundreds of smaller vessels (........). There venous blood (--------) from the oryx's nasal passages, cooled by respiratory evaporation, lowers the arterial blood temperature. Arrows indicate direction of blood flow. A brain cooler than the body temperature may be vital to desert survival.
Source: Taylor (1969)
Selective cooling of the brain to less than 41°C, which was 2.9°C below body temperature, has been demonstrated in sheep, goats and gazelle (Baker and Hayward, 1968; Taylor and Lyman, 1972; Degen, 1977c). In the goat, an additional source of heat exchange can be provided by the horn, which is the only superficial area that has a major drainage through the cavernous sinus (Taylor, 1966). There does not appear to be any information on the possible thermo-regulatory function of the horns of other tropical ruminants.
Counter-current cooling also occurs in the scrotum, because spermatogenesis is suppressed at normal rectal temperatures. The combination of dropping the testes away from the body and coiling the artery round the scrotal veins results in a temperature of the testes about 6°C below the core temperature of a ram in an ambient temperature of 21°C (Waites and Moule, 1961).
The effect of excess water intake is to raise body water turnover. This excess is rarely caused by drinking but is commonly caused by very green forage (sections 3.3.1 and 3.3.2). Lack of water is associated with dry forage and an inadequate watering regime. It obviously causes a reduction in body water turnover, but the way it does so is of interest.
For the watering regime to be adequate for ungulates eating dry forage, the following criteria must be met: the degree of dehydration must not exceed the temporary water holding capacity of the alimentary tract; the animal must have enough time to drink its fill; and the frequency of watering must be such as to prevent body water loss from reaching the stage of clinical dehydration.
In general, ruminants can replace 15-20% of their bodyweight at the first drink and 20-25% within 1-2.5 hours (section 3.3.1). The capacity and speed of fluid replacement appears to be higher in the more arid-adapted animals. For example, Saharan camels, weighing presumably about 450 kg, tolerate a loss of 100 I body water and replace it within 7-30 minutes (Gauthier-Pilters, 1974). Haired sheep and goats can drink up to 24% of their hydrated bodyweight in a few minutes. Dehydration does not reach a critical level in Indian desert sheep until they have lost 30% of their bodyweight, which is the stage at which Bedouin goats stop eating (Taneja, 1965; Shkolnik et al, 1972; More and Sahni, 1978; Williamson and Payne, 1978; C.R. Field, unpublished).
The donkey has a depressed appetite at 20% dehydration, but can restore all the water lost in 2-5 minutes. The ingested water floods the whole alimentary tract right up to the anal sphincter (Schmidt-Nielsen, 1965; Maloiy, 1970).
The effects of water restriction are felt mainly in the areas of energy production (section 4.1) and thermoregulation (section 4.2).
Where food is freely available a reduction in water intake reduces food intake. For example, when access to water was reduced to 1 h at 48 h and 72 h intervals, zebu oxen reduced their consumption of chaffed hay to 94% and 92% of their intake when water was available all the time. Their water consumption also decreased to 88% and 69% respectively of the original intake. All decreases were highly significant. There was no significant decrease in the starch equivalent consumed on 48 h watering, despite the 6% reduction in DMI. The reason was that more of the crude fibre fraction of the hay was digested than when water was freely available. There was, however, a significant lowering in energy intake when watering frequency was reduced to once every 72 h (French, 1956b).
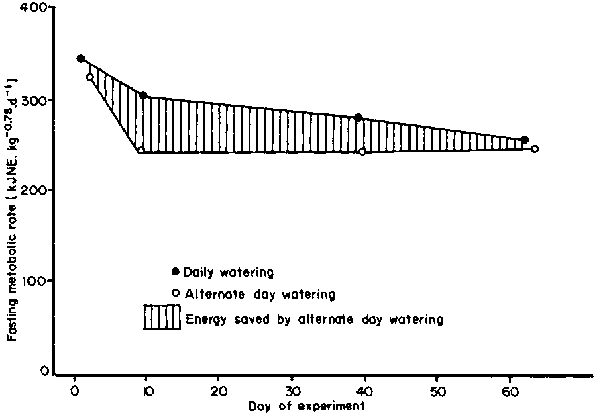
When fed a ration which provided only half their maintenance needs, zebu cattle on alternate day watering suffered no restriction of appetite compared with those on daily watering. But the reduction in their fasting metabolic rate to two thirds the value at maintenance (section 5.1.2) occurred more rapidly (Figure 18). The resultant energy saving, due to the quicker drop in demand, was estimated at 66 MJ NE (95 MJ ME) less than in cattle on daily watering (Finch and King, 1979). This work needs repeating because of the limited number of points on the graph.
Figure 18. The effects of daily and alternate day watering on the metabolic rate of zebus walking 16 km. d-1 on a half maintenance ratio. (Source: Finch and King (1979))
When the quality of grazing is low, cattle voluntarily restrict their intake and turnover of water, thereby controlling their nitrogen balance and achieving protein maintenance on diets which would normally be below maintenance requirements (Payne, 1963). The reason is that, on a low nitrogen diet, a high water intake and high urine volume flush urea out of the plasma so that it is not available for re-use in protein nor to stimulate microbial digestion of crude fibre. Thus differences in performance between animals fed a low nitrogen diet, or those grazing pastures low in nitrogen, may be partly related to differences in nitrogen metabolism caused by differences in water intake (Vercoe, 1971).
Lack of residual water for cooling and its effect on the ruminant, notably on thermolability and endogenous heat production have already been discussed in sections 4.2.6 and 4.1.3 respectively.
Donkeys are relatively unaffected by a water loss of 12 to 15% of their bodyweight. Food intake decreases by 27% at 15% dehydration, but this decrease is associated with an increase in apparent digestibility from 41 to 51% due to a longer retention time, particularly in the large intestine, which favours microbial digestion. Evaporative water loss also falls by about 50%, notably through the skin, and this is associated with an increase in respiratory rate and range of rectal temperature by 2 to 5.2°C. At between 15 and 30% dehydration, appetite is depressed and hence water loss through faecal output is decreased to 20% of the fully hydrated levels (Schmidt-Nielsen, 1965; Bullard et al, 1970; Maloiy, 1970; 1972).
Figure 19 provides a model of ungulate water needs in relation to climate and forage. It integrates the factors discussed above and illustrated in Figures 6,7 and 8.
The core of the model is the animal's body temperature, shown in the centre of the figure. This temperature must be maintained above a lower critical level to support respiratory function and metabolism, and below an upper critical limit to prevent heat death. The main source of heat gain at night is endogenous coming from intermediary metabolism, which is shown on the left half of the figure. The body temperature is above that of the environment and heat is lost from the skin by conduction, convection and radiation. This sensible heat exchange is illustrated in the middle of the right-hand segment of the figure. During the day, the sun heats up the animal and its environment, as shown at the top of the figure, causing a net inflow of heat to the body. Sensible heat loss may no longer be enough to control body temperature and the animal has to draw on residual water, surplus to metabolic requirements, for additional cooling. The body water cycle is illustrated in the bottom right-hand corner of the figure, and the contribution of sweating and panting to total heat loss is above it and to the left. A lack of residual water for cooling will cause a reduction in the contribution of sweating/panting to total heat loss, thereby allowing the body temperature to rise. The effect will be twofold: (a) to increase the temperature gradient at the skin and hence the sensible heat loss; and (b) to reduce activity and forage intake and hence the metabolic heat contribution to total body heat gain. The two feedback mechanisms combine to reduce residual body heat and hence body temperature.