
Animal health

4 June 2024, 17:00 hours; Rome
Overview
Situation: Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A virus that is spreading globally through human-to-human transmission but has also demonstrated ability to infect multiple animal species (from Bovidae, Canidae, Cebidae, Cercopithecidae, Cervidae, Cricetidae, Felidae, Hominidae, Hyaenidae, Mustelidae, Procionidae, Viverridae, Hippopotamidae, Myrmecophagidae, Atelidae, Rhinocerotidae, Suidae, Agamidae, Phasianidae, Anatidea and Castoridae and Muridae families) with spillover potential from one animal species to another. In rare occasions, spill-back from animals to humans has been evidenced (mink-to-human in the Netherlands [reference] and in Denmark [reference]; hamster-to-human in Hong Kong Special Administrative Region (China) [reference]).
Reported human cases: As of 19 May 2024, there have been 775 522 404 confirmed cases of COVID-19 including 7 049 617 deaths reported to WHO. In the last seven days, 36 014 new human cases and 321 deaths were reported worldwide. Since the beginning of the pandemic in March 2020, 232countries, states, and territories reported COVID-19 human cases across five geographic regions including Africa (57), the Americas (55), Asia (46), Europe (50), and Oceania (24) [reference]. Cumulative COVID-19 cases reported in humans globally are presented in Map 1. For detailed information on human cases, please refer to WHO COVID-19 Dashboard and WHO COVID-19 Weekly Updates.
Countries and territories with reported findings in animals (virological findings)1: France, Switzerland, Hong Kong SAR (China), Belgium, Netherlands, Germany, Russia, United States of America, Denmark, Japan, United Kingdom of Great Britain and Northern Ireland, Chile, Canada, Brazil, Sweden, Italy, Spain, South Africa, Greece, Argentina, Lithuania, Mexico, Slovenia, Estonia, Bosnia and Herzegovina, Latvia, Poland, Portugal, Puerto Rico, Croatia, Thailand, Uruguay, Myanmar, Indonesia, Singapore, Colombia, Finland, India, Ecuador, Egypt, Viet Nam, Senegal, Nigeria, Bulgaria, Hungary, Mongolia and Peru.
1 in order of first reported occurrence.
Situation in animals
Map 1 shows SARS-CoV-2 events2 in animals up to 4 June 2024 at the national level over an estimated cumulative COVID-19 human cases distribution map. Circles indicate countries reporting positive events in animals; circle size is proportional to the number of events reported in each country (see legend). The background layer map includes cumulative number of COVID-19 human cases according to WHO, 2022.
2 Events include animal cases officially reported by national authorities and the WOAH, or positive findings referred to in scientific publications.
Map. Results of published SARS-CoV-2 events in animals up to 4 June 2024 at national level, over a cumulative COVID-19 human cases background map
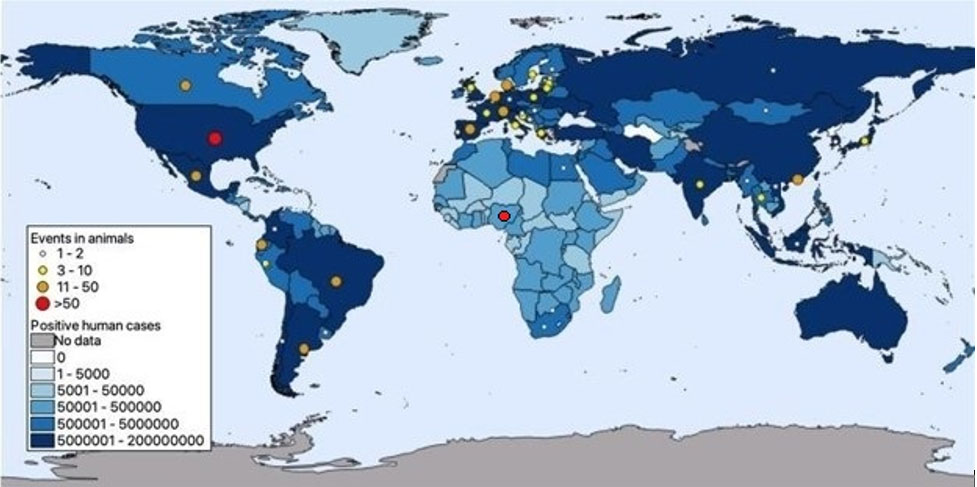
Table 1. Animal species naturally infected (RNA detection) by SARS-CoV-2
| Animal species | Scientific name | Country/Territory | Site | Year reported & number of epidemiological units affected (individual animal cases or production or marketing units such as farms or markets) |
|---|---|---|---|---|
|
Domestic cat |
Felis catus |
Argentina, Belgium, Brazil, Canada, Chile, Croatia, Ecuador, Egypt, Estonia, France, Finland, Germany, Greece, Hong Kong SAR., Hungary, Iran, Italy, Japan, Latvia, Mexico, Netherlands, Nigeria, Portugal, Republic of Korea, Russia, Spain, Switzerland, Thailand, United Kingdom of Great Britain and Northern Ireland, United States of America, Uruguay |
Household |
2020 (75) |
|
Domestic Dog |
Canis lupus familiaris |
Argentina, Bosnia and Herzegovina, Brazil, Canada, Chile, Colombia, Croatia, Denmark, Ecuador, Egypt, Finland, France, Hong Kong SAR, India, Italy, Japan, Jersey, Mexico, Myanmar, Netherlands, Nigeria, Portugal, Republic of Korea, Spain, Switzerland, Thailand, the United Kingdom of Great Britain and Northern Ireland, United States of America, Uruguay |
Household |
2020 (76) |
|
Domestic American Mink |
Neovison vison |
Bulgaria, Canada, Denmark, France, Greece, Italy, Latvia, Lithuania, Netherland, Poland, Spain, Sweden |
Farm |
2020 (349) |
|
Domestic Ferret |
Mustela furo |
Slovenia, United States of America |
Household |
2020 (1) |
|
Wild American Mink |
Neovison vison |
Spain, United States of America |
Free range |
2020 (no data) |
|
Western lowland Gorilla |
Gorilla gorilla gorilla |
the Netherlands, Spain, United States of America |
Zoo |
2021 (10) |
|
White-tailed deer |
Odocoileus virginianus |
Canada, United States of America |
Natural Park |
2021 (350) |
|
Binturong |
Arctictis binturong |
United States of America |
Zoo |
2021 (1) |
|
Coatimundi |
Nasua nasua |
Brazil, United States of America |
Zoo |
2021 (3) |
|
Fishing cat |
Prionailurus viverrinus |
United States of America |
Zoo |
2021 (1) |
|
Tiger |
Panthera tigris |
Argentina, Denmark, Indonesia |
Animal sanctuary |
2020 (1) |
|
Lion |
Panthera leo |
Croatia, Colombia, Estonia, Japan, the Netherlands, Puerto Rico, Singapore, South Africa, Spain, Sweden, United States of America |
Zoo |
2020 (2) |
|
Puma |
Puma concolor |
Argentina, South Africa, United States of America |
Wild animal exhibitor facility |
2020 (2) |
|
Snow Leopard |
Panthera uncia |
United States of America |
Zoo |
2020 (3) |
|
Indian Leopard |
Panthera pardus fusca |
India |
Free range |
2021 (1) |
|
Canada Lynx |
Lynx canadensis |
United States of America |
Zoo |
2021 (1) |
|
Spotted hyenas |
Crocuta crocuta |
United States of America |
Zoo |
2021 (2) |
|
Asian small-clawed otters |
Aonyx cinereus |
United States of America |
Aquarium |
2021 (9) |
|
Hamster |
Unspecified |
Hong Kong, SAR |
Pet shop |
2022 (2) |
|
Wild Eurasian River Otter |
Lutra lutra |
Spain |
Free range |
2021 (1) |
|
Hippopotamus |
Hippopotamus amphibius |
Belgium, Viet Nam |
Zoo |
2021 (1) |
|
Black-Tailed Marmoset |
Mico melanurus |
Brazil |
Free range |
2022 (1) |
|
Mule deer |
Odocoileus hemionus |
United States of America |
Natural Park |
2022 (1) |
|
Antillean manatees |
Trichechus manatus manatus |
Brazil |
Captive |
2020 (2) |
|
Giant anteater |
Myrmecophaga tridactyla |
Brazil |
Free range |
2022 (1) |
|
Mandrill |
Mandrillus sphinx |
United States of America |
Zoo |
2022 (1) |
|
Monkey Squirrel |
Saimiri sciureus |
United States of America |
Zoo |
2022 (1) |
|
Red fox |
Vulpes vulpes |
Switzerland |
Zoo |
2022 (1) |
|
Cattle |
Unspecified |
India, Nigeria, Republic of Korea |
Animal-rearing pockets |
2021/2022 (32) |
|
Buffalo |
Unspecified |
India |
Animal-rearing pockets |
2021/2022 (13) |
|
Goat |
Unspecified |
Nigeria |
Unspecified |
2021/2022 (46) |
|
Black-and brown headed Spider Monkey |
Ateles fusciceps |
Ecuador |
Captive |
2022 (20) |
| Common woolly monkey | Lagothrix lagothricha | Ecuador | Captive | 2022 (1) |
|
White rhinoceros |
Ceratotherium simum |
Senegal |
Natural reserve |
2023 (1) |
|
Ducka |
Unspecified |
Nigeria |
Households and backyard farms |
2021/2022 (2) |
|
Chickena |
Unspecified |
Nigeria |
Households and backyard farms |
2021/2022 (10) |
|
Turkeya |
Unspecified |
Nigeria |
Households and backyard farms |
2021/2022 (1) |
|
Sheep |
Unspecified |
Nigeria |
Households and backyard farms |
2021/2022 (50) |
|
Pig |
Unspecified |
Nigeria |
Households and backyard farms |
2021/2022 (4) |
|
Lizard |
Agama agama |
Nigeria |
Households and backyard farms |
2021/2022 (19) |
|
Eurasian beaver |
Castor fiber |
Mongolia |
Farm |
2021 (1) |
|
White-fronted capuchin |
Cebus unicolor |
Peru |
Captive |
2022/2023 (9)b |
|
House mouse |
Mus musculus |
Mexico |
Urban |
2020 (4) |
|
Brown rat |
Rattus norvegicus |
Mexico |
Urban |
2020 (3) |
Source: WOAH WAHIS, country reports and peer-reviewed journals3. Please see the respective articles under section “recent publications”.
3 Information from preprints is not included in this table.
a These are the first reports of viral RNA being detected in avian species though published experimental challenge studies have not indicated host susceptibility.
b Pool of nine samples.
Table 2. Animal species susceptibility to SARS-CoV-2 based on experimental infection studies
| Animal species | Scientific name (wild animals) | Susceptibility | Transmission to co-housed animals of same species |
|---|---|---|---|
|
Raccoon dogs (reference) |
Nyctereutes procyonoides |
Yes |
Yes |
|
Red Fox (reference) |
Vulpes vulpes |
Yes |
Not specified |
|
Coyotes |
Canis latrans |
No |
- |
|
Deer mice (reference) |
Peromyscus maniculatus |
Yes |
Yes |
|
Bank voles (reference) |
Myodes glareolus |
Yes |
No |
|
Bushy-tailed woodrats (reference) |
Neotoma cinerea |
Yes |
Not specified |
|
Laboratory BALB/c mice (reference) |
|
Yes |
Yes |
|
White-tailed deer (reference) |
Odocoileus virginianus |
Yes |
Yes |
|
Ferret (reference) |
Mustela furo |
Yes |
Yes |
|
Egyptian fruit bat |
Rousettus aegyptiacus |
Yes |
Yes |
|
Striped skunks (reference) |
Mephitis mephitis |
Yes |
Not specified |
|
Zebra fish (reference) |
Danio rerio |
Yes |
Not specified |
|
Zebra mussel (reference1) (reference2) |
Dreissena polymorpha |
Yes |
Not specified |
|
Syrian hamsters |
Mesocricetus auratus |
Yes |
Yes |
|
Tree shrews (reference1) (reference2) |
Tupaia belangeri chinensis |
Yes |
Not specified |
|
Rhesus macaques (reference) |
Macaca mulatta |
Yes |
Not specified |
|
The crab-eating macaque (reference) |
Macaca fascicularis |
Yes |
Not specified |
|
Baboons (reference) |
Papio hamadryas |
Yes |
Not specified |
|
Common marmosets (reference) |
Callithrix jacchus |
Yes |
Not specified |
|
Cynomolgus macaques (reference) |
Macaca fascicularis |
Yes |
Not specified |
|
African green monkeys (reference) |
Chlorocebus aethiops |
Not susceptible |
Not specified |
|
Mosquitoes (reference1) (reference2) |
Aedes aegypti, Aedes. albopictus, Culex tarsalis and Culex quinquefasciatus |
Not susceptible |
- |
|
Midge (reference) |
Culicoides sonorensis |
Not susceptible |
- |
|
Chicken – Duck – Geese – Turkey – Quail and Pigeon (reference) |
- |
Not susceptible |
- |
|
Pig (reference1) (reference2) (reference3) |
- |
Yes (Low susceptibility) |
No |
|
Cattle (reference1) (reference2) (reference3) |
- |
Yes (Low susceptibility) |
No |
|
Horse (reference) |
- |
No |
- |
|
Sheep (reference) |
- |
Yes (Low susceptibility) |
No1 |
|
Goat (reference1) (reference2) |
- |
Yes (Low susceptibility) |
Not specified |
|
Alpaca (reference) |
- |
No |
- |
|
Rabbit (reference) |
- |
Yes |
Not specified |
| Cat (reference) | - | Yes | Yes |
|
Dog (reference) |
- |
Yes (Low susceptibility) |
No |
|
Sprague Dawley rats (reference) |
Rattus norvegicus |
Yes |
Not specified |
|
Elk (reference1) (reference2) |
Cervus canadensis |
Yes (ancestral virus) |
No |
|
Mule deer (reference) |
Odocoileus hemionus |
Yes |
Yes |
|
Mexican free-tailed bats (reference) |
Tadarida brasiliensis |
Yes |
No |
1 Though RNA detected in some in-contact animals but none of them seroconverted.
Vaccination in animals
- The Toronto Zoo, Canada, has administered Zoetis vaccine to nearly 150 animals including primates, big cats, swine, bats and Mustelidae. Other zoos in Canada such as the Assiniboine Park Zoo in Winnipeg, Manitoba Province have also carried out vaccination in susceptible animals. [reference 1; reference 2]
- An experimental vaccination study was performed in juvenile cats using a spike protein-based subunit SARS-CoV-2 vaccine. The two adjuvanted vaccine formulations protected juvenile cats against virus shedding from the upper respiratory tract and viral replication in the lower respiratory tract and hearts. [reference]
- The Finnish Fur Breeders’ Association (FIFUR) and the University of Helsinki developed the Furcovac vaccine to allow for a vaccination roll out in mink farms, targeting about 50 000 animals. The Finnish Food Safety Authority provided a conditional license for the vaccine. [reference]
- A COVID-19 vaccine for animals is being developed jointly by Applied DNA Sciences and Evvivax. [reference]
- The United States based veterinary pharmaceutical company Zoetis has developed a COVID-19 inactivated vaccine uniquely formulated for animal species. The vaccine has already been used in multiple zoos in -United States since 2021 targeting multiple animal species including great apes, tigers, cheetahs, snow leopards, mountain lions, ferrets, black bears, and grizzly bears, among others. [reference 1; reference 2]
- Zoetis published a study assessing vaccine efficacy in cats and dogs [reference]. Vaccines were efficacious in mounting an immune response as judged by the generation of serum neutralizing antibodies in-vitro
- Zoetis has started the process of donating around 26,000 doses of its COVID-19 vaccine for animals to zoos and animal sanctuaries in 13 countries, including the United States and Canada [reference]
- The Federal Center for Animal Health (FGBI ARRIAH) in the Russian Federation developed an inactivated vaccine named Carnivac-Cov that targets cats, dogs, minks, and foxes [reference]. Several countries worldwide have already held negotiations with the manufacturer for the registration and supply of the drug to their countries [reference 1; reference 2; reference 3].
Recent publications
Agusi, E. R., Schön, J., Allendorf, V., Eze, E. A., Asala, O., [...], & Meseko, C. A. (2024). SARS-CoV and SARS-CoV-2 cross-reactive antibodies in domestic animals and wildlife in Nigeria suggest circulation of sarbecoviruses. One Health, 100709. [reference]. This study detected antibodies against SARS-CoV and SARS-CoV-2 in sera collected from dogs, rabbits and Pangolins using ELISA, a subset of ELISA positive sera confirmed by to be positive by virus neutralization test or indirect immunofluorescence assays
Boggiatto, P. M., Buckley, A., Cassmann, E. D., Seger, H., Olsen, S. C., & Palmer, M. V. (2024). Persistence of viral RNA in North American elk experimentally infected with an ancestral strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Scientific Reports, 14(1). [reference]. This experimental infection study demonstrated that elk do not develop clinical signs of disease following inoculation with SARS-CoV-2, but they do develop a neutralizing antibody response. Additionally, SARS-CoV-2 RNA detected in the medial retropharyngeal lymph nodes of infected elk three weeks after experimental infection.
Carvajal, M., Saenz, C., Fuentes, N., Guevara, R., Muñoz, E., Prado-Vivar, B., [...], & Barragan, V. (2024). SARS-CoV-2 infection in brown-headed spider monkeys (Ateles fusciceps) at a wildlife rescue center on the coast of Ecuador—South America. Microbiology Spectrum, 12(4). [reference]. This study reports SARS-CoV-2 infection of four sick brown-headed spider monkeys (Ateles fusciceps) at a wildlife rescue center in Ecuador after exposure to COVID-19 workers.
Lee, L. K. F., Himsworth, C. G., Prystajecky, N., Dibernardo, A., Lindsay, L. R., Albers, T. M., Dhawan, R., Henderson, K., Mulder, G., Atwal, H. K., Beattie, I., Wobeser, B. K., Parsons, M. H., & Byers, K. A. (2024). SARS-CoV-2 Surveillance of Wild Mice and Rats in North American Cities. Ecohealth, Volume 21, 1–8. [reference]. Two brown rats and 11 house mice were positive for SARS-CoV-2 neutralizing antibodies using a surrogate virus neutralization test, but negative or indeterminate with the Multiplexed Fluorometric ImmunoAssay COVID-Plex. Oro-nasopharyngeal swabs and fecal samples tested negative by RT-qPCR, with an indeterminate fecal sample in one house mouse.
Martínez-Hernández, F., Gonzalez-Arenas, N. R., Cervantes, J. a. O., Villalobos, G., Olivo-Diaz, A., Rendon-Franco, E., Maravilla, P., Valdovinos, M. R., & Muñoz-Garcia, C. I. (2024). Identification of SARS-CoV-2 in urban rodents from Southern Mexico City at the beginning of the COVID-19 pandemic. Revista Do Instituto De Medicina Tropical De São Paulo, 66. [reference]. SARS-CoV-2 RNA detected in intestinal samples of Four mice (12.1%) and three rats (5.8%) trapped along a water channel of a public park as part of a pest control program in Mexico, at the beginning of the COVID-19 pandemic, during the fall and winter of 2020.
Sandoval-Ramírez, C. M., Ballesteros, N., Pinilla, J. C., Hernández, C., Muñoz, M., & Ramírez, J. D. (2024). SARS-CoV-2 Mu variant in dogs visiting veterinary clinics during the third pandemic peak in Eastern Colombia. Veterinary Research Communications, Online ahead of print. [reference]. This study detected infection of domestic dogs (Canis lupus familiaris) with the Mu variant of SARS-CoV-2, the variant with the most death burden during the whole pandemic in Colombia. The infected dogs presented mild and reversible respiratory and gastrointestinal symptoms, or no clinical manifestations at all.
Tan, C. S., Adrus, M., Rahman, S. P. H., Azman, H. I. M., & Abang, R. a. A. (2024). Seroevidence of SARS-CoV-2 spillback to rodents in Sarawak, Malaysian Borneo. BMC Veterinary Research, 20(1). [reference]. This retrospective study tested 208 archived plasma from rodents in Malaysia collected between from 2018 to 2022 to detect neutralising antibodies against SARS-CoV-2 using a surrogate virus neutralisation test, and detected two seropositive rodents (Sundamys muelleri and Rattus rattus), which were sampled in 2021 and 2022, respectively.
FAO publications
- GLEWS+ Risk Assessment entitled SARS-CoV-2 in animals used for fur farming published on 20 January 2021. This Tripartite Risk Assessment evaluates the risk of introduction and spread of SARS-CoV-2 within fur farming systems as well as whether farmed fur animals could play a significant role in the spread of SARS-CoV-2 to humans via spillover. The publication has been translated in other UN languages (Arabic, Chinese, French, Russian, Spanish).
- COVID-19 and animals: Information on risk mitigation measures for livestock and agricultural professionals published on 08 January 2021. In response to the growing concern caused by the new SARS-CoV-2 variant strain related to minks, the guidelines raise awareness amongst livestock professionals about highly susceptible captive farmed wild or domestic species and provide practical guidelines on how to prevent the infection of animals or get infected.
- Supplementary tables which summarize the predicted susceptibility of ~500 animal species to SARS-CoV-2, based on the available studies on ACE2. This have been included in the publication Exposure of humans or animals to SARS-CoV-2 from wild, livestock, companion and aquatic animals published in July 2020 and its summary.
- Recommendations for the epidemiological investigation of SARS-CoV-2 in exposed animals, with a supplementary document Investigating potential recombination of MERS-CoV and SARS-CoV-2 or other coronaviruses in camels, both published in November 2021.
- Guidelines to mitigate the impact of the COVID-19 pandemic on livestock production and animal health published in 2020: Describe the impact of COVID-19 on livestock production and animal disease prevention and control and provide practical recommendations for actors along value chains to reduce this impact and ensure continuity of the livestock supply chain and animal health.
- Impact of COVID-19 on the delivery of veterinary services and animal disease reporting published in 2021: Survey to understand how the COVID-19 pandemic may have affected the routine activities of animal disease reporting and surveillance for early detection in the countries using EMA-i (Event Mobile Application).
FAO actions
Global level
- The coronavirus network (COVINET) meeting will be organized on 26-27 March in Geneva. FAO and representatives from the their reference centers on zoonotic coronaviruses will attend the meeting.
- FAO, WHO, WOAH and UNEP have organized the Quadripartite Global Technical Meeting on MERS-CoV and Other Emerging Zoonotic Coronaviruses, held from 27-29 November in Riyadh, Kingdom of Saudi Arabia.
- FAO takes part in regular WHO virus evolution group meetings to discuss latest findings on SARS-CoV-2 variants of interest and variants of concern.
- FAO attended the WHO technical workshop on regional surveillance of pathogens with epidemic and pandemic potential in mink from the WHO European Region: lessons learned and way forward, organized from 29 to 30 March 2023, Copenhagen, Denmark.
- The FAO/WOAH advisory group on SARS-CoV-2 evolution in animals held a call on the 9th of May 2023 to discuss the cryptic SARS-CoV-2 lineages detected in some mink farms.
- FAO has designated FAO Reference Centres for Zoonotic Coronaviruses. To date, Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe) and Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise Giuseppe Caporale (IZSAM), Italy (joint center), the Federal State-Financed Institution “The Federal Center for Animal Health” (FSVPS-FGBI ARRIAH), Russian Federation , CSIRO Australian Centre for Diseases Preparedness (ACDP), Australia, and Ohio State University Infectious Diseases Institute (OSU-IDI), USA, Institut de recherche pour le développement (IRD) and Centre de coopération internationale en recherche agronomique pour le développement (CIRAD) of France (joint center), and Ministry for Primary Industries, New Zealand have been appointed. Furthermore, Friedrich-Loeffler-Institut (FLI),Germany designated as Reference Centre for Emerging Zoonotic Pathogens and High Biosecurity/ Biocontainment Facilities.
- On 7 March 2022, the Tripartite Organization (FAO/ WHO/WOAH) issued a joint statement on the prioritization of monitoring SARS-CoV-2 infection in wildlife and preventing the formation of animal reservoirs. [reference]
- FAO established a Letter of Agreement with Hong Kong University for MERS-CoV and SARS-CoV-2 testing and capacity building for project countries.
- The Joint FAO/IAEA Centre (Nuclear Techniques in Food and Agriculture) (CJN) is working, through its veterinary laboratory network in 69 countries, to support diagnosis of SARS-CoV-2 in animals and monitoring of virus contamination in the environment.
Regional and national level
Within FAO’s COVID-19 Recovery and Response Programme, several projects are already in place as part of Preventing the Next Zoonotic Pandemic (PNP), that help countries to better prevent and mitigate risks related to SARS-CoV-2 at the animal-human interface and build national capacities in pandemic preparedness (see below for details).
Regional level
- FAO Regional Office for Asian and the Pacific (RAP):
- FAO produced a video about the COVID-19 response in Bangladesh, highlighting the collective support of the United Nations and partners under the leadership of the Government of Bangladesh. Thanks to the long partnership with USAID in addressing pandemic threats at source, FAO utilized its expertise to improve community-level case detection and risk mitigation during the public health emergency. USAID also funded the COVID-19 response project in Bangladesh. Watch the video here.
- Strengthening regional capacities to address COVID-19 impacts on animal health sector in East and Southeast Asia [April 2020 – September 2022]
- FAO ECTAD in Nepal has been monitoring of the sample collection for the companion animals for the testing of SARS-CoV-2, and providing technical guidance for sample collection, sample preservation and testing via ELISA. Training for laboratory testing of SARS-CoV-2 will be conducted in early 2023, followed by testing of collected samples in CVL.
- FAO ECTAD Indonesia with Directorate of Animal Health, Disease Investigation Center (DIC) in Subang and West Java provincial government continued risk profiling and sample collection of SARS-CoV-2 among civets in West Java province. Cross-sectoral meeting between national steering committee for One Health and stakeholders was conducted on 10-11 November 2022, and agreed to conduct epidemiology-laboratory analysis on information of civets and civet owners.
- FAO ECTAD Indonesia conducted an online meeting with Disease Investigation Centre (DIC) in Subang to discuss about the SARS-CoV-2 diagnosis from animal samples by PCR testing algorithm. Positive samples will be tested for whole genome sequencing in Illumina, whilst negative samples will be tested for Pan-coronavirus using PREDICT protocol. All positive samples will be also tested using nanopore technology at DIC Wates. Provision on nanopore reagents is ongoing.
- FAO Regional Office for Africa (RAF):
- Strengthening regional capacities to address negative impacts of COVID-19 on the animal health sector in Africa [June 2020 - June 2022].
- Support project for COVID-19 case detection and emergency response along livestock value chains in Cameroon [July 2020 - March 2021].
Through these projects and others, FAO is supporting countries in West and Central Africa since the beginning of the pandemic in mitigating negative impacts of COVID-19 by:
- Strengthening national animal disease surveillance systems through joint risk assessments at the animal-human-environment interface and trainings on field investigation, sample collection, shipment and transportation [since October 2020].
- Improving COVID-19 testing and reinforcing veterinary laboratories as effective tools for the detection of animal diseases. For instance, in Ghana, national veterinary laboratories actively supported public health laboratories in COVID-19 testing. FAO ECTAD Ghana also supported the installation of a Laboratory Information Management System (SILAB/LIMS) One Health module at the Accra Veterinary Laboratory to improve management of COVID-19 samples [since October 2020].
- Assisting the Governments such as in Cameroon to secure a Technical Cooperation Programme project to support COVID-19 detection and emergency response along the livestock value chain in Cameroon [since July 2020].
National level
- Cameroon:
- COVID-19 cases detection and reporting in livestock value chain actors in North-West and South-West regions, Cameroon [February – April 2021]
- Support project for COVID-19 cases detection and emergency response along livestock value chain in Cameroon [July 2020 – March 2021].
- Risk communication and community engagement plan for COVID-19 control, was developed and validated [March 2021].
- Cote d’Ivoire:
- As part of the surveillance for COVID-19, FAO supported the Department of Veterinary Services (DVS) in the detection, investigation, and follow-up testing of SARS-CoV-2 in domestic animals in contact with COVID-19 patients. Four veterinary clinics were involved in sample collection from animals received in care. A total of 52 samples were collected in four veterinary clinics and all were tested negative at Pasteur Institute of Cote d'Ivoire (IPCI) [September 2021].
- In collaboration with the national One Health Platform FAO conducted a joint risk assessment using a One Health approach (MoL , MoH and MoE) on COVID-19 and Highly Pathogenic Avian Influenza [October 2021].
- Ghana:
- A training was held for staff of the Accra Veterinary Laboratory on dissemination of existing biosecurity and biosafety guidelines and standard operating procedures for SARS-CoV-2 testing in Veterinary Laboratories [May 2021].
- Provision of 11 000 units of filtering face piece (FFP3) respirators on 3 December 2020 to the Ministry of Health in response to a request from the Government of Ghana, which aimed to protect health workers in the Ghana Health Service from COVID-19. This donation came at a time when the country was facing a second wave of the COVID-19 pandemic [December 2020].
- A two-day advocacy awareness training was held for 30 multi-disciplinary stakeholders in the food value chains on the impacts of the COVID 19 pandemic on food security/nutrition and livelihoods [March 2021].
- A three-day training workshop “Training of bushmeat traders, hunters and wildlife exporters in Kumasi and Accra to create awareness on COVID-19 and other related Priority Zoonotic Diseases (PZDs) of wildlife with potential to spill over to humans from bush meat traders and consumers” was held in collaboration with the Veterinary Service Directorate (VSD) of the Ministry of Food and Agriculture (MOFA), Wildlife Division of the Forestry Commission, Food and Drugs Authority (FDA), Environmental Health Department (EHD), Ghana Health Service, and Ghana Police Service (GPS) [April 2021]
- Improving COVID-19 testing by reinforcing veterinary laboratories (see under ‘Regional level’).
- Guinea: A total of 1 116 biological samples from 244 animals of various species (bat, rodent, swine and small ruminants) in 13 human-animal-environment interfaces were collected and all tested negative for Ebola and Marburg virus as well as for SARS-CoV-2 [May - June 2021].
- Liberia: Training and mentorship of staff on Biosafety and Biosecurity including COVID-19 bio-risk management [March 2022].
- Nigeria:
- A study was conducted to evaluate COVID-19-related risk communication; results indicated no COVID-19 related risk communication messaging was conducted with animal health professionals, even though there are efforts for other zoonoses. A suggestion was made to engage the One Health Risk Communication pillar to address this deficiency [June - July 2021].
- A workshop was held to discuss biosecurity and biosafety; guidelines and SOPs for COVID-19 were disseminated and discussed to establish a unified set of national guidelines [July 2021].
- Senegal:
- In collaboration with the national One Health Platform FAO conducted a joint risk assessment using a One Health approach (MoL , MoH and MoE) on Covid-19 and Highly Pathogenic Avian Influenza [February - April 2021].
- Surveillance and research activities at human-wildlife-livestock-ecosystem interface and their results were mapped and shared during a workshop with the OH platform to enhance multisectoral collaboration [June 2020 - July 2021].
- Guidelines elaborated by the National Park Direction were updated and disseminated, including Information, Education and Communication (IEC) materials for various audiences and stakeholders [February - April 2021].
- National Veterinary Laboratory readiness for SARS-COV-2 testing was assessed using the FAO Laboratory Mapping tools (LMT-Core, LMT-safety modules and LMT-COVID) and a Biorisk assessment was conducted, ensuring that capacities of laboratories are built to handle (collect, transport, store) samples with appropriate levels of biosecurity and biosafety of SARS-COV-2 testing [February - April 2021].
- Mali, Mauritania, Niger, and Senegal: IESA - Mitigating the effects of Covid-19 on pastoral communities in West Africa [October 2020 – October 2022].
- Papua New Guinea: Emergency support to prevent and mitigate the impact of COVID-19 along the agricultural value chain [April 2021 – March 2022].
- Rwanda: TCPF: Support to Fostering the One Health Operationalization in Rwanda [May 2021 – April 2023].
- China: Emergency response to mitigate the impact of coronavirus (COVID-19) on the most vulnerable persons in rural areas in China [March 2020 – December 2022]
- Sultanate of Oman: Understanding and mitigating the risks of SARS-CoV-2 transmission from COVID-19 human patients to in-contact farmed and companion animals [June 2021 – December 2022].
- Collection of serum samples from 617 animals including camels, cattle, sheep and goats has been completed in June 2022, and the sera were submitted to the virology laboratory of Hong Kong University for testing using surrogate virus neutralization test; positive results (if any) will be confirmed by another assay.
- The laboratory testing was completed in August 2022 at the virology laboratory of the Hong Kong University. Sera of six animals, representing four species (cattle, camel, sheep, and goat), tested positive by both surrogate virus neutralization test and the plague reduction neutralization test 50.
- United Arab Emirates:
- Detection of potential recombination of MERS-CoV with SARS-CoV-2 or other Coronaviruses in dromedary camels [October 2021 – April 2022].
- Understanding and mitigating the risks of SARS-CoV-2 transmission from COVID-19 human patients to in-contact farmed and companion animals [December 2021 - December 2022].
- The laboratory testing was completed in September 2022 at the animal health laboratory of the Abu Dhabi Agriculture and Food Safety Authority (ADAFSA). Sera of 13 animals, representing three species (sheep, goats, and captive gazelles), had positive ELISA results, however, none of them tested positive by the surrogate virus neutralization test. All collected nasal swab samples tested negative for SARS-CoV-2 RNA by RT-PCR. However, all collected sera of camels, tested for MERS CoV-Ab using ELISA, revealed that 164 of the 181 samples were positive (90.6%).
Disclaimer
Information provided herein is current as of the date of issue. Information added or changed since the last SARS-COV-2 animal situation update appears in orange. Human cases are depicted in the geographic location of their report. For some cases, exposure may have occurred in one geographic location but reported in another. For cases with unknown onset date, reporting date was used instead. FAO compiles information drawn from multiple national (Ministries of Agriculture or Livestock, Ministries of Health; Centers for Disease Prevention and Control [CDC]) and international sources (World Health Organization [WHO], World Organisation for Animal Health [WOAH]) as well as peer-reviewed scientific articles and preprints. FAO makes every effort to ensure, but does not guarantee, accuracy, completeness or authenticity of the information. The boundaries and names shown and the designations used on these map(s) do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries. Dashed lines on maps represent approximate border lines for which there may not yet be full agreement.
Contact
If interested in a previous issue please send an email to EMPRES-Animal Health specifying the intended use of the document.
