Registration by equivalence *
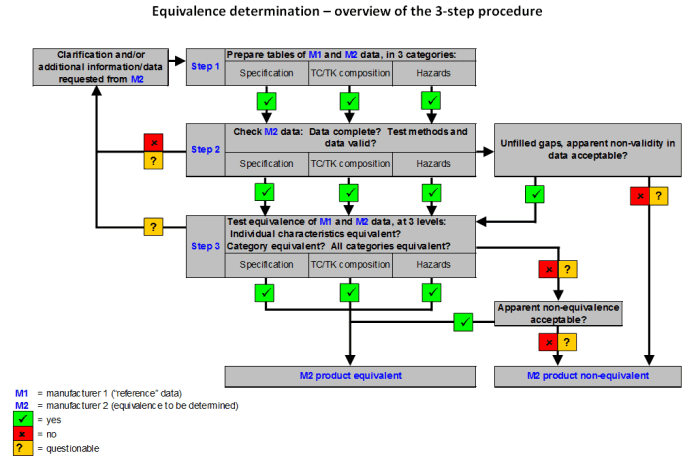
Registration based on equivalence may be conducted if the pesticide submitted for registration can be shown to be equivalent to a similar pesticide that has already been registered in the country. Two pesticides are considered equivalent if the impurity and toxicological profiles, as well as the physical and chemical properties, of the technical materials originating from different manufacturers are similar, and therefore can be expected to present similar levels of risk.
The objective of equivalence determination is to determine whether or not the product of a second manufacturer is not worse (ref. quality and hazards) than the already registered product of a first manufacturer, on which the “reference” specification is based. If that can be proven, the product of the second manufacturer can also be registered.
Equivalence determination for microbial pest control agents follows a different procedure from chemical pesticides following a procedure which is summarized here. *
Registration by equivalence, if applicable, requires less data to be submitted by the applicant than for a complete local evaluation. Equivalence is a simple concept but its determination is rather complex and requires, in particular, expertise in pesticide product chemistry.
Detailed guidance on equivalence determination can be found in Chapter 3.2 of the Manual on development and use of FAO and WHO specifications for pesticides and its amendments.
Practical examples of how to conduct an equivalence evaluation are provided in the Training Manual published by WHO and FAO.