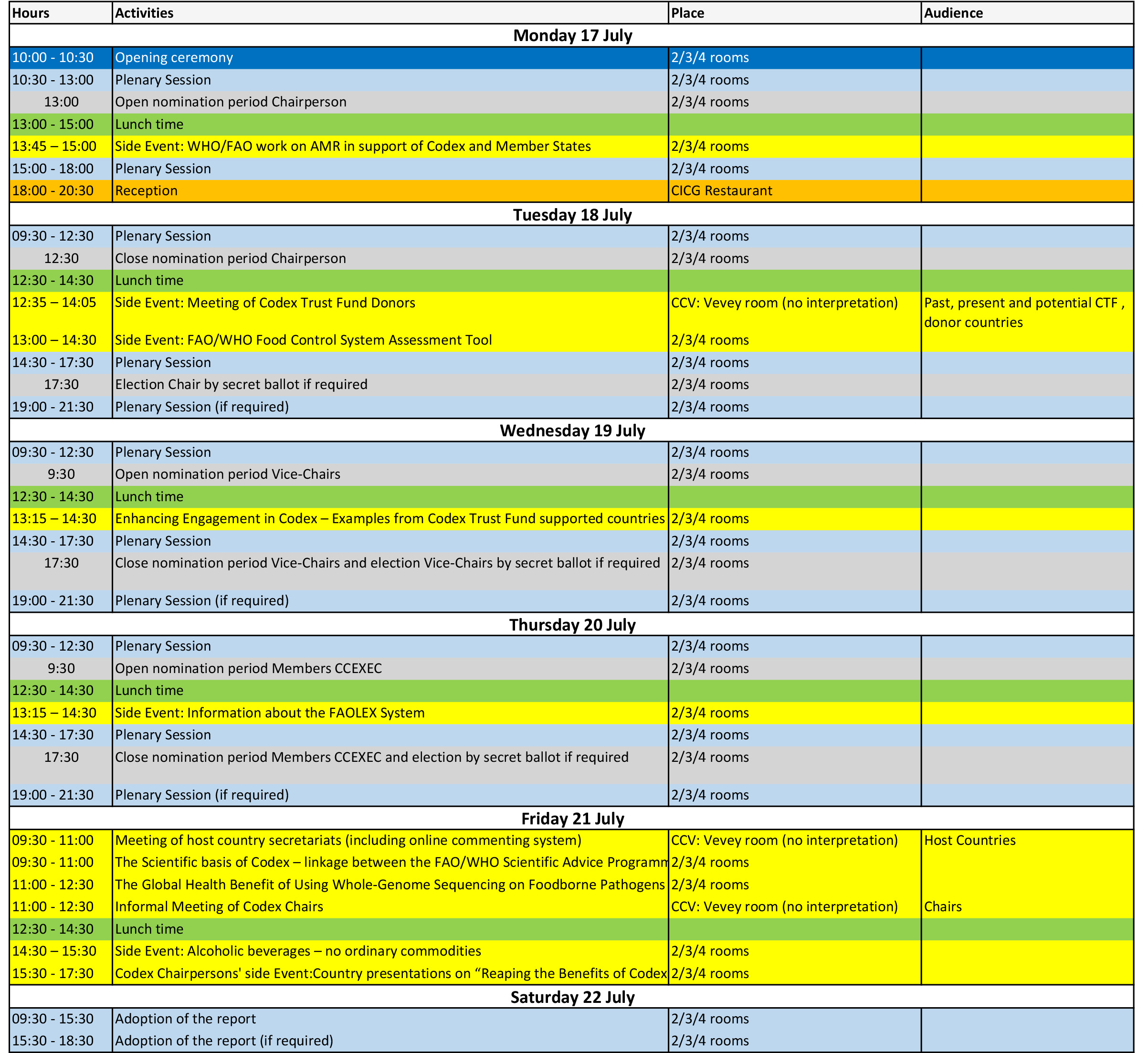
| 1 | 暂定议程 | 27/03/2017 | | CX/CAC 17/40/1 |
| 12.2 | 食品法典委员会、执行委员会及附属机构报告提出的事项
抗菌素耐药性实体工作组报告
| 06/03/2017 | | CX/CAC 17/40/12/Add.2 |
| 18 | 指定负责任命食典委附属机构主席的国家 | 31/03/2017 | | CX/CAC 17/40/21 |
| 17 | 选举主席、副主席和按地理区域选出的
执委会成员以及任命协调员和采用其他表决方式
| 28/03/2017 | | CX/CAC 17/40/20 Rev.1 |
| 6 | 在步骤5通过的食典文本 | 04/05/2017 | | CX/CAC 17/40/5 |
| 9 | 终止工作 | 02/05/2017 | | CX/CAC 17/40/9 |
| 7 | 法典文本的废除 | 02/05/2017 | | CX/CAC 17/40/7 |
| 5 | 食典文本的最终通过 | 04/05/2017 | | CX/CAC 17/40/3 |
| 16 | 食品法典委员会与其他国际组织之间的关系 | 18/05/2017 | | cx/cac 17/40/19 |
| 5.1 | 食典文本的最终通过 | 22/05/2017 | | CX/CAC 17/40/3 Add.1 |
| 8 | 新工作提案 | 19/05/2017 | | CX/CAC 17/40/8 |
| 14 | 粮农组织/世卫组织对食典委的科学支持:活动及今后工作报告 | 02/06/2017 | | CX/CAC 17/40/14 |
| 13 | 食典委秘书处:预算和财务事项 | 14/06/2017 | | Cx/CAC 17/40/13 |
| 4 | 《程序手册》修正案 | 12/06/2017 | | CX/CAC 17/40/2 |
| 12 | 食典委、执行委员会及各附属机构报告提出的事项 | 15/06/2017 | | CX/CAC 17/40/12 |
| 11 | 定期审查食典工作管理情况:电子工作组 | 19/06/2017 | | CX/CAC 17/40/11 |
| 14.2 | 粮农组织/世卫组织对食典委的科学支持:预算和财务事项 | 20/06/2017 | | CX/CAC 17/40/15 |
| 6.1 | 在步骤5通过的食典文本 | 19/06/2017 | | Cx/CAC 17/40/5 Add.1 |
| 7.1 | 法典文本的废除 | 19/06/2017 | | CX/CAC 17/40/7 Add.1 |
| 9.1 | 终止工作 | 19/06/2017 | | CX/CAC 17/40/9 Add.1 |
| 5.2 | 食典文本的最终通过 | 20/06/2017 | | CX/CAC 17/40/3 Add2 |
| 10 | 法典标准及相关文本修正 | 30/06/2017 | | CX/CAC 17/40/10 |
| 12.1 | 食典委、执行委员会及各附属机构报告提出的事项 | 04/07/2017 | | CX/CAC 17/40/12-Add.1 |
| 14.1 | 粮农组织/世卫组织对食典委的科学支持:抗菌素耐药性最新情况 | 04/07/2017 | | CX/CAC 17/40/14Add1 |
| 8.1 | 下文列出了制定新标准和相关文本的提案清单,包括相关报告中项目文件的参考信息。请食典委结合执委会开展的严格审查结果决定是否逐项开展新工作,并决定由哪个附属机构或其他机构开展新工作。请食典委根据《2014-2019年战略计划》和《确定工作重点及建立附属机构的标准》,审议这些提案。 | 12/07/2017 | | CX/CAC 17/40/8Add1 |
| 15.2 | 粮农组织和世卫组织提出的事项:能力发展活动 | 17/07/2017 | | CX/CAC 17/40/17 |
| 15.4 | Matters Arising from FAO and WHO: Codex Trust Fund
Activities | 17/07/2017 | | CX/CAC 17/40/18Add1 |
| 15.1 | 粮农组织和世卫组织提出的事项:政策和相关事项 | 17/07/2017 | | CX/CAC 17/40/16 |
| 2 | Report of the 72nd Session of the Executive Committee | 14/07/2017 | | REP17/EXEC1 |
| 2.1 | 食品法典委员会执行委员会
第七十三届会议报告
| 14/07/2017 | | REP17/EXEC2 |
| 15.3 | Matters Arising from FAO and WHO: Codex Trust Fund
Activities | 17/07/2017 | | CX/CAC 17/40/18 |
| 报 告 | 18/09/2017 | | REP17/CAC |
| 3.1 | Report of the FAO/WHO Coordinating Committee for Asia | 14/07/2017 | | REP17/ASIA |
| 101 | Communication from IAEA | 30/06/2017 | | CAC/40 INF/01 |
| 103 | Communication from IOC | 30/06/2017 | | CAC/40 INF/03 |
| 104 | Communication from IPPC | 05/07/2017 | | CAC/40 INF/04 |
| 105 | Communication from ISO | 30/06/2017 | | CAC/40 INF/05 |
| 106 | Communication from OIV | 30/06/2017 | | CAC/40 INF/06 |
| 107 | Communication from OECD | 30/06/2017 | | CAC/40 INF/07 |
| 108 | Communication from UNECE | 30/06/2017 | | CAC/40 INF/08 |
| 109 | Communication from OIE | 30/06/2017 | | CAC/40 INF/09 |
| 102 | Communication from IDF | 07/07/2017 | | CAC/40 INF/02 |
| 111 | Communication from WTO SPS | 10/07/2017 | | CAC/40 INF/11 |
| 201 | Division of Competence between the European Union and its Member States according to Rule of Procedure II Paragraph 5 of the Codex Alimentarius | 03/07/2017 | | CAC/40 CRD/01 |
| 112 | Communication from FAO (FAO GM Foods Platform) | 14/07/2017 | | CAC/40 INF/12 |
| 3 | Report of the FAO/WHO Coordinating Committee for Africa | 14/07/2017 | | REP17/AFRICA |
| 3.2 | Report of the FAO/WHO Coordinating Committee for Europe | 14/07/2017 | | REP17/EURO |
| 3.3 | Report of the FAO/WHO Coordinating Committee for Latin America and the Caribbean | 14/07/2017 | | REP17/LAC |
| 3.5 | Report of the FAO/WHO Coordinating Committee for North America and South West Pacific | 14/07/2017 | | REP17/NASWP |
| 3.4 | Report of the FAO/WHO Coordinating Committee for Near East | 14/07/2017 | | REP17/NE |
| 223 | Comments of Costa Rica on Item 19 | 15/07/2017 | | CAC/40 CRD/23 |
| 228 | Requests of Chile on item 19 | 18/07/2017 | | CAC/40 CRD/28 |
| 110 | Communication from STDF | 07/09/2017 | | CAC/40 INF/10 Rev2 |
| 4.1 | Comments - Amendments to the Procedural Manual | 28/06/2017 | | CX/CAC 17/40/2 Add.1 |
| 5.3 | Comments - Final Adoption of Codex Texts | 28/06/2017 | | CX/CAC 17/40/4 |
| 5.4 | Comments(by emails)- Final Adoption of Codex Texts | 30/06/2017 | | CX/CAC 17/40/4 Add.1 |
| 6.2 | Comments - Adoption of Codex Texts at Step 5 | 28/06/2017 | | CX/CAC 17/40/6 |
| 6.3 | Comments(by emails) - Adoption of Codex Texts at Step 5 | 30/06/2017 | | CX/CAC 17/40/6 Add.1 |
| 209 | Item 11: Comments of India | 13/07/2017 | | CAC/40 CRD/09 |
| 211 | Item 12.2: Comments of African Union, Tanzania and Health for Animals | 13/07/2017 | | CAC/40 CRD/11 |
| 212 | Item 14: Comments of Health for animals | 13/07/2017 | | CAC/40 CRD/12 |
| 213 | Comments of Gambia on Items 3, 4, 5, 6, 7, 8, 10 | 14/07/2017 | | CAC/40 CRD/13 |
| 214 | Comments of Papua New Guinea on Items 8, 11, 12, 15.3, 19 | 14/07/2017 | | CAC/40 CRD/14 |
| 216 | Comments of IFFO on Item 5 | 14/07/2017 | | CAC/40 CRD/16 |
| 217 | Comments of Cameroon on Items 5, 6, 7, 8, 10, 12.2 | 14/07/2017 | | CAC/40 CRD/17 |
| 218 | Comments of Indonesia on Items 4, 5, 6, 7, 8 | 14/07/2017 | | CAC/40 CRD/18 |
| 220 | Comments of Nicaragua on Item 8 | 15/07/2017 | | CAC/40 CRD/20 |
| 222 | Comments of Dominican Republic on Items 4, 5, 6, 7, 11, 12 | 15/07/2017 | | CAC/40 CRD/22 |
| 219 | Comments of Ghana on Items 4, 5, 6, 7, 8 | 15/07/2017 | | CAC/40 CRD/19 |
| 221 | Comments of Ecuador on Items 4, 5, 6, 7, 8, 9, 10, 11, 12 | 15/07/2017 | | CAC/40 CRD/21 |
| 224 | Comments of Panama on Item 2 | 15/07/2017 | | CAC/40 CRD/24 |
| 215 | Comments of Chile on Items 5, 11 | 14/07/2017 | | CAC/40 CRD/15 |
| 204 | Item 6: Comments of Colombia, India, Nigeria, Tanzania, African Union | 12/07/2017 | | CAC/40 CRD/04 |
| 205 | Item 7: Comments of Philippines, Tanzania, African Union | 03/07/2017 | | CAC/40 CRD/05 |
| 206 | Item 8: Comments of Nigeria, Philippines, Tanzania, African Union | 03/07/2017 | | CAC/40 CRD/06 |
| 203 | Item 5: Comments of Ecuador, Qatar, Iran, Nicaragua, Nigeria, African Union, El salvador, Tanzania and INDIA | 13/07/2017 | | CAC/40 CRD/03 |
| 207 | Item 9: Comments of the Philippines | 13/07/2017 | | CAC/40 CRD/07 |
| 208 | Item 10: Comments of the Philippines, Malaysia, African Union and Tanzania | 13/07/2017 | | CAC/40 CRD/08 |
| 210 | Item 12.1: USA, Tanzania. African Union, Brazil, Costa Rica, Cuba, Ecuador, Egypt, Japan, Kenya, Mexico, Philippines and IDF | 13/07/2017 | | CAC/40 CRD/10 |
| 202 | Item 4: Comments of India, Tanzania and African Union | 13/07/2017 | | CAC/40 CRD/02 |
| 225 | Comments of the United States on Item 12.1 | 15/07/2017 | | CAC/40 CRD/25 |
| 226 | Comments of Nicaragua on Item 11 | 15/07/2017 | | CAC/40 CRD/26 |
| 227 | Comments of National Health Federation (NHF) on Items 3, 5 | 17/07/2017 | | CAC/40 CRD/27 |
| 230 | Comments of International Dairy Federation (IDF) on item 12 | 18/07/2017 | | CAC/40 CRD/30 |
| 229 | Comments of Senegal on items 5, 6, 7 | 18/07/2017 | | CAC/40 CRD/29 |
| 231 | Comments of Dominica of item 5 | 19/07/2017 | | CAC/40 CRD/31 |
| 232 | Commnets of Republique du Benin on item 4,5,6,7,8,10,12 | 19/07/2017 | | CAC/40 CRD/32 |
| 233 | Comments of Russian Federation on items 5, 6, 8, 11 | 20/07/2017 | | CAC/40 CRD/33 |
| 234 | Comments of Codex Trust Fund Secretariat on item 15.3 | 20/07/2017 | | CAC/40 CRD/34 |
| 235 | All files zip collection | | | |